Summary
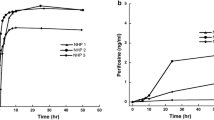
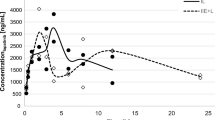
Purpose. Emerging evidence suggests that 5' Adenosine Monophosphate-Activated Protein Kinase (AMPK), a key regulator of cellular bioenergetics, is a novel target for the treatment of glioblastoma (GBM), a lethal brain tumor. SBI-0206965, an aminopyrimidine derivative, is a potent AMPK inhibitor being investigated for the treatment of GBM. Here we characterized the systemic and brain pharmacokinetics (PK) and hepatic metabolism of SBI-0206965. Methods. We performed intracerebral microdialysis to determine brain partitioning of SBI-0206965 in jugular vein cannulated rats. We assessed systemic PK of SBI-0206965 in rats and C57BL/6 mice following oral administration. Employing human, mouse, and rat liver microsomes we characterized the metabolism of SBI-0206965. Results. SBI-0206965 is quickly absorbed, achieving plasma and brain extracellular fluid (ECF) peak levels within 0.25 – 0.65 h. Based on the ratio of Cmax and AUC in brain ECF to plasma (corrected for protein binding), brain partitioning is ~ 0.6—0.9 in rats. However, the compound has a short elimination half-life (1–2 h) and low relative oral bioavailability (~ 0.15). The estimated in-vitro hepatic intrinsic clearance of SBI-0206965 in mouse, rat and human was 325, 76 and 68 mL/min/kg, respectively. SBI-0206965 metabolites included desmethylated products, and the metabolism was strongly inhibited by ketoconazole, a CYP3A inhibitor. Conclusion. SBI-0206965 has adequate brain permeability but low relative oral bioavailability which may be due to rapid hepatic metabolism, likely catalyzed by CYP3A enzymes. Our observations will facilitate further development of SBI-0206965, and/or other structurally related molecules, for the treatment of GBM and other brain tumors.



Similar content being viewed by others
Data availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS (2020) CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro Oncol 22(12 Suppl 2):iv1-iv96. https://doi.org/10.1093/neuonc/noaa200
Johnson DR, O’Neill BP (2012) Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol 107(2):359–364. https://doi.org/10.1007/s11060-011-0749-4
National Brain Tumor Society website (2022) Quick Brain Tumor Facts. https://braintumor.org/brain-tumor-information/brain-tumor-facts/. Accessed 18 Mar 2022
Bonini MG, Gantner BN (2013) The multifaceted activities of AMPK in tumor progression–why the “one size fits all” definition does not fit at all? IUBMB Life 65(11):889–896. https://doi.org/10.1002/iub.1213
Dasgupta B, Chhipa RR (2016) Evolving Lessons on the Complex Role of AMPK in Normal Physiology and Cancer. Trends Pharmacol Sci 37(3):192–206. https://doi.org/10.1016/j.tips.2015.11.007
Zadra G, Batista JL, Loda M (2015) Dissecting the Dual Role of AMPK in Cancer: From Experimental to Human Studies. Mol Cancer Res 13(7):1059–1072. https://doi.org/10.1158/1541-7786.MCR-15-0068
Liu X, Chhipa RR, Pooya S, Wortman M, Yachyshin S, Chow LM, Kumar A, Zhou X, Sun Y, Quinn B, McPherson C, Warnick RE, Kendler A, Giri S, Poels J, Norga K, Viollet B, Grabowski GA, Dasgupta B (2014) Discrete mechanisms of mTOR and cell cycle regulation by AMPK agonists independent of AMPK. Proc Natl Acad Sci USA 111(4):E435–E444. https://doi.org/10.1073/pnas.1311121111
Chhipa RR, Fan Q, Anderson J, Muraleedharan R, Huang Y, Ciraolo G, Chen X, Waclaw R, Chow LM, Khuchua Z, Kofron M, Weirauch MT, Kendler A, McPherson C, Ratner N, Nakano I, Dasgupta N, Komurov K, Dasgupta B (2018) AMP kinase promotes glioblastoma bioenergetics and tumour growth. Nat Cell Biol 20(7):823–835. https://doi.org/10.1038/s41556-018-0126-z
Dite TA, Langendorf CG, Hoque A, Galic S, Rebello RJ, Ovens AJ, Lindqvist LM, Ngoei KRW, Ling NXY, Furic L, Kemp BE, Scott JW, Oakhill JS (2018) AMP-activated protein kinase selectively inhibited by the type II inhibitor SBI-0206965. J Biol Chem 293(23):8874–8885. https://doi.org/10.1074/jbc.RA118.003547
Egan DF, Chun MG, Vamos M, Zou H, Rong J, Miller CJ, Lou HJ, Raveendra-Panickar D, Yang CC, Sheffler DJ, Teriete P, Asara JM, Turk BE, Cosford ND, Shaw RJ (2015) Small Molecule Inhibition of the Autophagy Kinase ULK1 and Identification of ULK1 Substrates. Mol Cell 59(2):285–297. https://doi.org/10.1016/j.molcel.2015.05.031
Deng J, Jiang P, Yang T, Huang M, Xie J, Luo C, Qi W, Zhou T, Yang Z, Zou Y, Gao G, Yang X (2019) β2-adrenergic receptor signaling promotes neuroblastoma cell proliferation by activating autophagy. Oncol Rep 42(4):1295–1306. https://doi.org/10.3892/or.2019.7266
Yamamoto BK, Pehek EA (1990) A neurochemical heterogeneity of the rat striatum as measured by in vivo electrochemistry and microdialysis. Brain Res 506(2):236–242. https://doi.org/10.1016/0006-8993(90)91256-g
Apparaju SK, Gudelsky GA, Desai PB (2008) Pharmacokinetics of gemcitabine in tumor and non-tumor extracellular fluid of brain: an in vivo assessment in rats employing intracerebral microdialysis. Cancer Chemother Pharmacol 61(2):223–229. https://doi.org/10.1007/s00280-007-0464-1
Dave N, Gudelsky GA, Desai PB (2013) The pharmacokinetics of letrozole in brain and brain tumor in rats with orthotopically implanted C6 glioma, assessed using intracerebral microdialysis. Cancer Chemother Pharmacol 72(2):349–357. https://doi.org/10.1007/s00280-013-2205-y
Arora P, Gudelsky G, Desai PB (2021) Gender-based differences in brain and plasma pharmacokinetics of letrozole in sprague-dawley rats: Application of physiologically-based pharmacokinetic modeling to gain quantitative insights. PLoS ONE 16(4):e0248579. https://doi.org/10.1371/journal.pone.0248579
Paxinos G, Watson CR, Emson PC (1980) AChE-stained horizontal sections of the rat brain in stereotaxic coordinates. J Neurosci Methods 3(2):129–149. https://doi.org/10.1016/0165-0270(80)90021-7
Naritomi Y, Terashita S, Kimura S, Suzuki A, Kagayama A, Sugiyama Y (2001) Prediction of human hepatic clearance from in vivo animal experiments and in vitro metabolic studies with liver microsomes from animals and humans. Drug Metab Dispos 29(10):1316–24. https://dmd.aspetjournals.org/content/29/10/1316
Hosea NA, Collard WT, Cole S, Maurer TS, Fang RX, Jones H, Kakar SM, Nakai Y, Smith BJ, Webster R, Beaumont K (2009) Prediction of human pharmacokinetics from preclinical information: comparative accuracy of quantitative prediction approaches. J Clin Pharmacol 49(5):513–533. https://doi.org/10.1177/0091270009333209
Chen A, Zhou X, Tang S, Liu M, Wang X (2016) Evaluation of the inhibition potential of plumbagin against cytochrome P450 using LC-MS/MS and cocktail approach. Sci Rep 6:28482. https://doi.org/10.1038/srep28482
Ahwazi D, Neopane K, Markby GR, Kopietz F, Ovens AJ, Dall M, Hassing AS, Gräsle P, Alshuweishi Y, Treebak JT, Salt IP, Göransson O, Zeqiraj E, Scott JW, Sakamoto K (2021) Investigation of the specificity and mechanism of action of the ULK1/AMPK inhibitor SBI-0206965. Biochem J 478(15):2977–2997. https://doi.org/10.1042/BCJ20210284
Zheng Y, Liu L, Wang Y, Xiao S, Mai R, Zhu Z, Cao Y (2021) Glioblastoma stem cell (GSC)-derived PD-L1-containing exosomes activates AMPK/ULK1 pathway mediated autophagy to increase temozolomide-resistance in glioblastoma. Cell Biosci 11(1):63. https://doi.org/10.1186/s13578-021-00575-8
Dower CM, Bhat N, Gebru MT, Chen L, Wills CA, Miller BA, Wang HG (2018) Targeted Inhibition of ULK1 Promotes Apoptosis and Suppresses Tumor Growth and Metastasis in Neuroblastoma. Mol Cancer Ther 17(11):2365–2376. https://doi.org/10.1158/1535-7163.MCT-18-0176
National Center for Biotechnology Information (2022) PubChem Compound Summary for CID 92044402. https://pubchem.ncbi.nlm.nih.gov/compound/sbi-0206965. Accessed 18 Mar 2022
Sarkaria JN, Hu LS, Parney IF, Pafundi DH, Brinkmann DH, Laack NN, Giannini C, Burns TC, Kizilbash SH, Laramy JK, Swanson KR, Kaufmann TJ, Brown PD, Agar N, Galanis E, Buckner JC, Elmquist WF (2018) Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol 20(2):184–191. https://doi.org/10.1093/neuonc/nox175
Choi GW, Lee YB, Cho HY (2019) Interpretation of Non-Clinical Data for Prediction of Human Pharmacokinetic Parameters: In Vitro-In Vivo Extrapolation and Allometric Scaling. Pharmaceutics 11(4):168. https://doi.org/10.3390/pharmaceutics11040168
FDA/CEDR resources page. Food and Drug Administration Web site. https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers#table1-2. Accessed 18 Mar 2022
FDA/CEDR resources page. Food and Drug Administration Web site. In Vitro Drug Interaction Studies - Cytochrome P450 Enzyme and Transporter-Mediated Drug Interactions Guidance for Industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/vitro-drug-interaction-studies-cytochrome-p450-enzyme-and-transporter-mediated-drug-interactions. Accessed 18 Mar 2022
Eagling VA, Tjia JF, Back DJ (1998) Differential selectivity of cytochrome P450 inhibitors against probe substrates in human and rat liver microsomes. Br J Clin Pharmacol 45(2):107–114. https://doi.org/10.1046/j.1365-2125.1998.00679.x
Mahatthanatrakul W, Sriwiriyajan S, Ridtitid W, Boonleang J, Wongnawa M, Rujimamahasan N, Pipatrattanaseree W (2021) Effect of cytochrome P450 3A4 inhibitor ketoconazole on risperidone pharmacokinetics in healthy volunteers. J Clin Pharm Ther 37(2):221–225. https://doi.org/10.1111/j.1365-2710.2011.01271.x
de Wildt SN, Kearns GL, Leeder JS, van den Anker JN (1999) Cytochrome P450 3A: ontogeny and drug disposition. Clin Pharmacokinet 37(6):485–505. https://doi.org/10.2165/00003088-199937060-00004
Claassen V (1994) Intraperitoneal Drug Administration. In: Techniques in the behavioral and neural sciences, Vol. 12C, Elsevier, pp 46–58
Lau CE, Ma F, Wang Y, Smith C (1996) Pharmacokinetics and bioavailability of midazolam after intravenous, subcutaneous, intraperitoneal and oral administration under a chronic food-limited regimen: relating DRL performance to pharmacokinetics. Psychopharmacology 26(3):241–248. https://doi.org/10.1007/BF02246454
Kruijtzer CM, Beijnen JH, Schellens JH (2002) Improvement of oral drug treatment by temporary inhibition of drug transporters and/or cytochrome P450 in the gastrointestinal tract and liver: an overview. Oncologist 7(6):516–530. https://doi.org/10.1634/theoncologist.7-6-516
Hendrikx JJ, Lagas JS, Rosing H, Schellens JH, Beijnen JH, Schinkel AH (2013) P-glycoprotein and cytochrome P450 3A act together in restricting the oral bioavailability of paclitaxel. Int J Cancer 132(10):2439–2447. https://doi.org/10.1002/ijc.27912
Sato S, Matsumiya K, Tohyama K, Kosugi Y (2021) Translational CNS Steady-State Drug Disposition Model in Rats, Monkeys, and Humans for Quantitative Prediction of Brain-to-Plasma and Cerebrospinal Fluid-to-Plasma Unbound Concentration Ratios. AAPS J 23(4):81. https://doi.org/10.1208/s12248-021-00609-6
Adhikari B, Li J, Brandel MG, Futalan D, Akers J, Deming T, Chen CC, Carter BS (2017) The use of TMZ embedded hydrogels for the treatment of orthotopic human glioma xenografts. J Clin Neurosci 45:288–292. https://doi.org/10.1016/j.jocn.2017.07.027
Acknowledgements
University of Cincinnati Brain Tumor Center (Pankaj B. Desai) and National Institutes of Health (NIH) partially funded this project grants R01NS099162 and R01NS114074 (Biplab DasGupta).
Funding
This project was partially funded by University of Cincinnati Brain Tumor Center (Pankaj B. Desai) and National Institutes of Health (NIH) grants R01NS099162 and R01NS114074 (Biplab DasGupta).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, design, material preparation, data collection and analysis. The first draft of the manuscript was written by Janki M. Desai and Pankaj B. Desai and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The experimental protocol for rat study was approved by the University of Cincinnati Institutional Animal Care and Use Committee (IACUC, Protocol No. 20–01-24–01). C57BL/6 mice were generated by in-house breeding at the Cincinnati Children’s Hospital Medical Center (CCHMC; Cincinnati, OH). Housing and experimental protocols employing C57BL/6 mice were approved by the CCHMC IACUC (Protocol No. IACUC2021-0031).
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Desai, J.M., Karve, A.S., Gudelsky, G.A. et al. Brain pharmacokinetics and metabolism of the AMP-activated protein kinase selective inhibitor SBI-0206965, an investigational agent for the treatment of glioblastoma. Invest New Drugs 40, 944–952 (2022). https://doi.org/10.1007/s10637-022-01278-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-022-01278-8