Summary
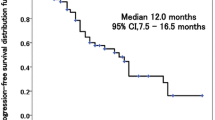
Objective Apatinib is an oral small molecule anti-angiogenic drug. This phase I study aimed to establish the feasible dose of apatinib in combination with pemetrexed plus carboplatin as first-line therapy for epidermal growth factor receptor (EGFR) and anaplasticlymphoma kinase (ALK) negative stage IV non-squamous non-small cell lung cancer (NSCLC). Methods Using a 3 + 3 dose-reduction design, patients received oral apatinib at four dose levels: 750 mg qd, 500 mg qd, 500 mg/day two weeks on/one week off schedule (500 mg schedule 2/1) or 250 mg qd. Pemetrexed (500 mg/m2) plus carboplatin (AUG = 5) was administered every three weeks. Maintenance therapy by apatinib or pemetrexed could be carried on until disease progression or unacceptable toxicity. The feasible dose was determined based on cycle 1 dose-limiting toxicities (DLT); other assessments included safety and antitumor activity according to response evaluation criteria in solid tumors. Result A total of twelve patients were enrolled and cycle 1 DLTs were observed in two patients at 750 mg qd dosage of apatinib (both Grade 3 hypertension), two patients at 500 mg qd (Grade 3 hypertension and Grade 3 hand-foot syndrome), and only one of six patients at 500 mg/day schedule 2/1 (Grade 3 hypertension). The most frequently drug-related adverse events (AEs) were hematological toxicity, hypertension, hand-foot syndrome, and hepatic transaminases elevation. Partial response was observed in four patients of eleven evaluable patients (objective response rate 36.4%), and six patients exhibited stable disease (disease control rate 90.9%). Conclusion In patients with advanced non-squamous NSCLC, the feasible dose of apatinib given with standard-dose pemetrexed and carboplatin was 500 mg/day schedule 2/1. The schedule was generally well tolerated and demonstrated promising clinical benefit in NSCLC.


Similar content being viewed by others
References
Jardim DL, Gagliato Dde M, Ribeiro KB, Shimada AK, Katz A (2012) Bevacizumab as first-line therapy in advanced non-small-cell lung cancer: a brazilian center experience. Drugs R&D 12(4):207–216
Crino L, Dansin E, Garrido P, Griesinger F, Laskin J, Pavlakis N, Stroiakovski D, Thatcher N, Tsai CM, Wu YL, Zhou C (2010) Safety and efficacy of first-line bevacizumab-based therapy in advanced non-squamous non-small-cell lung cancer (SAiL, MO19390): a phase 4 study. Lancet Oncol 11(8):733–740
Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S, Feng J, He J, Han B, Wang J, Jiang G, Hu C, Zhang H, Cheng G, Song X, Lu Y, Pan H, Zheng W, Yin AY (2015) Beyond: a randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or Placebo in Chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol 33(19):2197–2204
Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, Manegold C (2010) Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL). Ann Oncol 21(9):1804–1809
Patel JD, Socinski MA, Garon EB, Reynolds CH, Spigel DR, Olsen MR, Hermann RC, Jotte RM, Beck T, Richards DA, Guba SC, Liu J, Frimodt-Moller B, John WJ, Obasaju CK, Pennella EJ, Bonomi P, Govindan R (2013) PointBreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol 31(34):4349–4357
Barlesi F, Scherpereel A, Gorbunova V, Gervais R, Vikstrom A, Chouaid C, Chella A, Kim JH, Ahn MJ, Reck M, Pazzola A, Kim HT, Aerts JG, Morando C, Loundou A, Groen HJ, Rittmeyer A (2014) Maintenance bevacizumab-pemetrexed after first-line cisplatin-pemetrexed-bevacizumab for advanced nonsquamous nonsmall-cell lung cancer: updated survival analysis of the AVAPERL (MO22089) randomized phase III trial. Ann Oncol 25(5):1044–1052
Paz-Ares LG, Biesma B, Heigener D, von Pawel J, Eisen T, Bennouna J, Zhang L, Liao M, Sun Y, Gans S, Syrigos K, Le Marie E, Gottfried M, Vansteenkiste J, Alberola V, Strauss UP, Montegriffo E, Ong TJ, Santoro A (2012) Phase III, randomized, double-blind, placebo-controlled trial of gemcitabine/cisplatin alone or with sorafenib for the first-line treatment of advanced, nonsquamous non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol 30(25):3084–3092
Laurie SA, Solomon BJ, Seymour L, Ellis PM, Goss GD, Shepherd FA, Boyer MJ, Arnold AM, Clingan P, Laberge F, Fenton D, Hirsh V, Zukin M, Stockler MR, Lee CW, Chen EX, Montenegro A, Ding K, Bradbury PA (2014) Randomised, double-blind trial of carboplatin and paclitaxel with daily oral cediranib or placebo in patients with advanced non-small cell lung cancer: NCIC clinical trials group study BR29. Eur J Cancer (Oxford, England : 1990) 50(4):706–712
Scagliotti GV, Vynnychenko I, Park K, Ichinose Y, Kubota K, Blackhall F, Pirker R, Galiulin R, Ciuleanu TE, Sydorenko O, Dediu M, Papai-Szekely Z, Banaclocha NM, McCoy S, Yao B, Hei YJ, Galimi F, Spigel DR (2012) International, randomized, placebo-controlled, double-blind phase III study of motesanib plus carboplatin/paclitaxel in patients with advanced nonsquamous non-small-cell lung cancer: MONET1. J Clin Oncol Off J Am Soc Clin Oncol 30(23):2829–2836
Tian S, Quan H, Xie C, Guo H, Lu F, Xu Y, Li J, Lou L (2011) YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci 102(7):1374–1380
Zhang H (2015) Apatinib for molecular targeted therapy in tumor. Drug Des Devel Ther 9:6075–6081
Mi YJ, Liang YJ, Huang HB, Zhao HY, Wu CP, Wang F, Tao LY, Zhang CZ, Dai CL, Tiwari AK, Ma XX, To KK, Ambudkar SV, Chen ZS, Fu LW (2010) Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res 70(20):7981–7991
Li Z, Shi M, Cheng H, Liu X, Jian PX, Chen G, Wei L, Liu W, Zhang Y, Kai LI (2012) A phase II, multicenter, placebo-controlled trial of apatinib in patients with advanced nonsquamous non-small cell lung cancer (NSCLC) after two previous treatment regimens. J Clin Oncol 30(15_suppl)
Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R, Wang Z, Wang Q, Ouyang X, Yang Y, Ba Y, Liang J, Lin X, Luo D, Zheng R, Wang X, Sun G, Wang L, Zheng L, Guo H, Wu J, Xu N, Yang J, Zhang H, Cheng Y, Wang N, Chen L, Fan Z, Sun P, Yu H, Randomized, Double-Blind, Placebo-Controlled Phase III (2016) Trial of Apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol Off J Am Soc Clin Oncol 34(13):1448–1454
Hu X, Zhang J, Xu B, Jiang Z, Ragaz J, Tong Z, Zhang Q, Wang X, Feng J, Pang D, Fan M, Li J, Wang B, Wang Z, Zhang Q, Sun S, Liao C (2014) Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer 135(8):1961–1969
Fang SC, Zhang HT, Zhang YM, Xie WP (2017) Apatinib as post second-line therapy in EGFR wild-type and ALK-negative advanced lung adenocarcinoma. Onco Targets Ther 10:447–452
Cheng H, Sun A, Guo Q, Zhang Y (2018) Efficacy and safety of apatinib combined with chemotherapy for the treatment of advanced gastric cancer in the Chinese population: a systematic review and meta-analysis. Drug Des Devel Ther 12:2173–2183
Shi Q, Guo X, Wang Z, Cheng X, Xia L, Li X, Hu W, Zhao F, Liu Y, Wang J (2017) P2.01-011 the efficiency and safety of Apatinib plus S-1 as second-line or laterline chemotherapy for advanced squamous cell lung carcinoma. J Thorac Oncol 12(11):S2073
Wu F, Zhang S, Yu J, Gao G, Li W, Cai W, Su C, Chen X, Ren S, Zhou C (2017) P3.01-085 a Phase 2 trial of Apatinib in advanced non-squamous NSCLC: updated data and clinical benefit of continuing Apatinib after initial progression. J Thorac Oncol 12(11):S2235
Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y, Sun G, Yang Y, Wang L, Xu N, Cheng Y, Wang Z, Zheng L, Tao M, Zhu X, Ji D, Liu X, Yu H (2013) Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol Off J Am Soc Clin Oncol 31(26):3219–3225
Ma JT, Sun J, Sun L, Zhang SL, Huang LT, Han CB (2018) Efficacy and safety of apatinib in patients with advanced nonsmall cell lung cancer that failed prior chemotherapy or EGFR-TKIs: a pooled analysis. Medicine 97(35):e12083
Zhang D, Zhang C, Huang J, Guan Y, Guo Q (2018) Clinical investigation of the efficacy and toxicity of apatinib (YN968D1) in stage III/IV non-small cell lung cancer after second-line chemotherapy treatment: a retrospective study. Thoracic Cancer 9:1754–1762
Zinner RG, Obasaju CK, Spigel DR, Weaver RW, Beck JT, Waterhouse DM, Modiano MR, Hrinczenko B, Nikolinakos PG, Liu J, Koustenis AG, Winfree KB, Melemed SA, Guba SC, Ortuzar WI, Desaiah D, Treat JA, Govindan R, Ross HJ (2015) PRONOUNCE: randomized, open-label, phase III study of first-line pemetrexed + carboplatin followed by maintenance pemetrexed versus paclitaxel + carboplatin + bevacizumab followed by maintenance bevacizumab in patients ith advanced nonsquamous non-small-cell lung cancer. J Thorac Oncol 10(1):134–142
Li J, Zhao X, Chen L, Guo H, Lv F, Jia K, Yv K, Wang F, Li C, Qian J, Zheng C, Zuo Y (2010) Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer 10:529
Lin C, Wang S, Xie W, Zheng R, Gan Y, Chang J (2016) Apatinib inhibits cellular invasion and migration by fusion kinase KIF5B-RET via suppressing RET/Src signaling pathway. Oncotarget 7(37):59236–59244
Zeng DX, Wang CG, Lei W, Huang JA, Jiang JH (2017) Efficiency of low dosage apatinib in post-first-line treatment of advanced lung adenocarcinoma. Oncotarget 8(39):66248–66253
Funding
The work was supported by the Department of Thoracic Oncology, Cancer Center, West China Hospital, Sichuan University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Meijuan Huang declares that she has no conflict of interest. Author Youling Gong declares that he has no conflict of interest. Author Jiang Zhu declares that he has no conflict of interest. Author Yi Qin declares that she has no conflict of interest. Author Feng Peng declares that she has no conflict of interest. Author Li Ren declares that she has no conflict of interest. Author Zhenyu Ding declares that he has no conflict of interest. Author Yongmei Liu declares that she has no conflict of interest. Author Chengzhi Cai declares that he has no conflict of interest. Author Yongsheng Wang declares that he has no conflict of interest. Author You Lu declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, M., Gong, Y., Zhu, J. et al. A phase I dose-reduction study of apatinib combined with pemetrexed and carboplatin in untreated EGFR and ALK negative stage IV non-squamous NSCLC. Invest New Drugs 38, 478–484 (2020). https://doi.org/10.1007/s10637-019-00811-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-019-00811-6