Summary
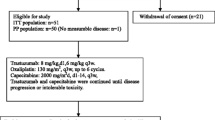
Objective To investigate the efficacy of bevacizumab and trastuzumab combined with docetaxel, oxaliplatin, and capecitabine (B-DOCT) as first-line treatment of advanced human epidermal growth factor receptor 2 (HER2)-positive gastric cancer (GC). Methods In this multicentre, single-arm, phase II study, tumor HER2 status was determined centrally prior to treatment. Patients with advanced HER2-positive adenocarcinoma of the stomach or gastroesophageal junction (immunohistochemistry 3+ or immunohistochemistry 2+/silver in-situ hybridization positive) were treated with six cycles of bevacizumab 7.5 mg/kg (day 1), docetaxel 50 mg/m2 (day 1), oxaliplatin 100 mg/m2 (day 1), capecitabine 850 mg/m2 b.i.d. (days 1–14), and trastuzumab 6 mg/kg (day 1) every three weeks, followed by maintenance with bevacizumab, capecitabine, and trastuzumab until disease progression. The primary objective was to demonstrate an improvement of progression-free survival (PFS) to >7.6 months (observed in the ToGA trial) determined according to the lower limit of the 95 % confidence interval (CI). Secondary endpoints were safety, objective response rate (ORR), and overall survival (OS). Results Twenty-five patients with HER2-positive tumors were treated with B-DOCT between March 2011 and September 2014. At a median follow-up of 17 months, median PFS was 10.8 months (95%CI: 9.0–NA), OS was 17.9 months (95%CI: 12.4–NA). One-year PFS and OS were 52 % and 79 %, respectively. The ORR was 74 % (95%CI: 52–90 %). Two patients became resectable during treatment with B-DOCT and achieved a pathological complete response. The most common treatment-related grade ≥ 3 adverse events were: neutropenia (16 %), diarrhoea (16 %), and hypertension (16 %). Conclusions B-DOCT is a safe and active combination in HER2-positive GC, supporting further investigations of DOC with HER2/vascular endothelial growth factor (VEGF) inhibition in HER2-positive GC.


Similar content being viewed by others
References
Wagner AD, Unverzagt S, Grothe W, et al. (2010) Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 3:CD004064. doi:10.1002/14651858.CD004064.pub3
Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 24:4991–4997. doi:10.1200/JCO.2006.06.8429
Wang J, Xu R, Li J, et al. (2015) Randomized multicenter phase III study of a modified docetaxel and cisplatin plus fluorouracil regimen compared with cisplatin and fluorouracil as first-line therapy for advanced or locally recurrent gastric cancer. Gastric Cancer. doi:10.1007/s10120-015-0457-4
Amarantidis K, Xenidis N, Chelis L, et al. (2011) Docetaxel plus oxaliplatin in combination with capecitabine as first-line treatment for advanced gastric cancer. Oncology 80:359–365. doi:10.1159/000330199
Di Lauro L, Vici P, Belli F, et al. (2014) Docetaxel, oxaliplatin, and capecitabine combination chemotherapy for metastatic gastric cancer. Gastric Cancer 17:718–724. doi:10.1007/s10120-013-0321-3
Deenen MJ, Meulendijks D, Boot H, et al. (2015) Phase 1a/1b study of docetaxel, oxaliplatin and capecitabine in patients with advanced cancer of the stomach or the gastro-esophageal junction. In press, Cancer Chemotherapy and Pharmacology
Rüschoff J, Hanna W, Bilous M, et al. (2012) HER2 testing in gastric cancer: a practical approach. Mod Pathol 25:637–650. doi:10.1038/modpathol.2011.198
Koopman T, Smits MM, Louwen M, et al. (2015) HER2 positivity in gastric and esophageal adenocarcinoma: clinicopathological analysis and comparison. J Cancer Res Clin Oncol 141:1343–1351. doi:10.1007/s00432-014-1900-3
Bang Y-J, Van Cutsem E, Feyereislova A, et al. (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–697. doi:10.1016/S0140-6736(10)61121-X
Van Cutsem E, Bang Y-J, Feng-Yi F, et al. (2014) HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. doi:10.1007/s10120-014-0402-y
Pegram MD, Konecny GE, O’Callaghan C, et al. (2004) Rational Combinations of Trastuzumab With Chemotherapeutic Drugs Used in the Treatment of Breast Cancer. J Natl Cancer Inst 96:739–749. doi:10.1093/jnci/djh131
Ding X, Qu X, Fan Y, et al. (2014) Trastuzumab and oxaliplatin exhibit a synergistic antitumor effect in HER2-postive gastric cancer cells. Anti-Cancer Drugs 25:315–322. doi:10.1097/CAD.0000000000000048
Ryu M-H, Yoo C, Kim JG, et al. (2015) Multicenter phase II study of trastuzumab in combination with capecitabine and oxaliplatin for advanced gastric cancer. Eur J Cancer 51:482–488. doi:10.1016/j.ejca.2014.12.015
Peng L, Zhan P, Zhou Y, et al. (2012) Prognostic significance of vascular endothelial growth factor immunohistochemical expression in gastric cancer: a meta-analysis. Mol Biol Rep 39:9473–9484. doi:10.1007/s11033-012-1812-8
Park DJ, Thomas NJ, Yoon C, Yoon SS (2015) Vascular endothelial growth factor a inhibition in gastric cancer. Gastric Cancer 18:33–42. doi:10.1007/s10120-014-0397-4
Konecny G, Meng Y, Untch M (2004) Association between HER-2/neu and vascular endothelial growth factor expression predicts clinical outcome in primary breast cancer patients. Clin Cancer Res 10:1706–1716
Singh R, Kim WJ, Kim P-H, Hong HJ (2013) Combined blockade of HER2 and VEGF exerts greater growth inhibition of HER2-overexpressing gastric cancer xenografts than individual blockade. Exp Mol Med 45:e52. doi:10.1038/emm.2013.111
Le X-F, Mao W, Lu C, et al. (2014) Specific blockade of VEGF and HER2 pathways results in greater growth inhibition of breast cancer xenografts that overexpress HER2. Cell Cycle 7:3747–3758. doi:10.4161/cc.7.23.7212
Sun Y, Dey N, Brammer M, et al. (2013) Bevacizumab confers additional advantage to the combination of trastuzumab plus pertuzumab in trastuzumab-refractory breast cancer model. Cancer Chemother Pharmacol 72:733–745. doi:10.1007/s00280-013-2233-7
Ohtsu A, Shah MA, Van Cutsem E, et al. (2011) Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 29:3968–3976. doi:10.1200/JCO.2011.36.2236
Van Cutsem E, de Haas S, Kang Y-K, et al. (2012) Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol 30:2119–2127. doi:10.1200/JCO.2011.39.9824
Coudert B, Pierga J-Y, Mouret-Reynier M-A, et al. (2014) Use of [(18)F]-FDG PET to predict response to neoadjuvant trastuzumab and docetaxel in patients with HER2-positive breast cancer, and addition of bevacizumab to neoadjuvant trastuzumab and docetaxel in [(18)F]-FDG PET-predicted non-responders (AVATAXHER): Lancet Oncol 15:1493–502. doi:10.1016/S1470-2045(14)70475-9
Eisenhauer EA, Therasse P, Bogaerts J, et al. (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. doi:10.1016/j.ejca.2008.10.026
Rüschoff J, Dietel M, Baretton G, et al. (2010) HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Arch 457:299–307. doi:10.1007/s00428-010-0952-2
Campigotto F, Weller E (2014) Impact of informative censoring on the Kaplan-Meier estimate of progression-free survival in phase II clinical trials. J Clin Oncol 32:3068–3074. doi:10.1200/JCO.2014.55.6340
Gianni L, Romieu GH, Lichinitser M, et al. (2013) AVEREL: a randomized phase III Trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. J Clin Oncol 31:1719–1725. doi:10.1200/JCO.2012.44.7912
Pierga J-Y, Petit T, Delozier T, et al. (2012) Neoadjuvant bevacizumab, trastuzumab, and chemotherapy for primary inflammatory HER2-positive breast cancer (BEVERLY-2): an open-label, single-arm phase 2 study. Lancet Oncol 13:375–384. doi:10.1016/S1470-2045(12)70049-9
Abbas O, Shamseddin A, Temraz S, Haydar A (2013) Posterior reversible encephalopathy syndrome after bevacizumab therapy in a normotensive patient. BMJ Case Rep 2013:3–6. doi:10.1136/bcr-2012-007995
Lyros E, Walter S, Keller I, et al. (2014) Subacute reversible toxic encephalopathy related to treatment with capecitabine: A case report with literature review and discussion of pathophysiology. Neurotoxicology 42C:8–11. doi:10.1016/j.neuro.2014.02.010
Kaneda H, Okamoto I, Satoh T, Nakagawa K (2012) Reversible posterior leukoencephalopathy syndrome and trastuzumab. Investig New Drugs 30:1766–1767. doi:10.1007/s10637-011-9696-3
Shen L, Li J, Xu J, et al. (2014) Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: randomized, double-blind, phase III study (AVATAR study). Gastric Cancer. doi:10.1007/s10120-014-0351-5
Tewari KS, Sill MW, Long HJ, et al. (2014) Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 370:734–743. doi:10.1056/NEJMoa1309748
Hurwitz H, Fehrenbacher L, Novotny W, et al. (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342. doi:10.1056/NEJMoa032691
Miller K, Wang M, Ph D, et al. (2007) Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 357:2666–2676. doi:10.1056/NEJMoa072113
Reck M, von Pawel J, Zatloukal P, et al. (2010) Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL). Ann Oncol 21:1804–1809. doi:10.1093/annonc/mdq020
Strong VE, Song KY, Park CH, et al. (2010) Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg 251:640–646. doi:10.1097/SLA.0b013e3181d3d29b
Miles DW, de Haas SL, Dirix LY, et al. (2013) Biomarker results from the AVADO phase 3 trial of first-line bevacizumab plus docetaxel for HER2-negative metastatic breast cancer. Br J Cancer 108:1052–1060. doi:10.1038/bjc.2013.69
Wilke H, Muro K, Van Cutsem E, et al. (2014) Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 15:1224–1235. doi:10.1016/S1470-2045(14)70420-6
Fuchs CS, Tomasek J, Yong CJ, et al. (2013) Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 6736:31–39. doi:10.1016/S0140-6736(13)61719-5
Qin S Phase III study of apatinib in advanced gastric cancer: A randomized, double-blind, placebo-controlled trial. J Clin Oncol 32:5 s, 2014 (suppl; abstr 4003).
Yoon HH, Bendell JC, Braiteh FS, et al. (2014) Ramucirumab (RAM) plus FOLFOX as front-line therapy (Rx) for advanced gastric or esophageal adenocarcinoma (GE-AC): Randomized, double-blind, multicenter phase 2 trial. J Clin Oncol 32(5 s); suppl; abstr 4004
Wang X, Chen X, Fang J, Yang C (2013) Overexpression of both VEGF-A and VEGF-C in gastric cancer correlates with prognosis, and silencing of both is effective to inhibit cancer growth. Int J Clin Exp Pathol 6:586–597
Yang X, Sun H-J, Li Z-R, et al. (2015) Gastric cancer-associated enhancement of von Willebrand factor is regulated by vascular endothelial growth factor and related to disease severity. BMC Cancer 15:1083. doi:10.1186/s12885-015-1083-6
Acknowledgments
We thank the patients who participated in this study and their families. We are very grateful to the participating centres and the contribution of all study coordinators, nurse practitioners, and data managers who helped performing this study. This study was funded with an unrestricted grant from Hoffmann-La Roche.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
This study was funded with an unrestricted grant from Hoffmann-La Roche. Author JWBG received personal fees from Hoffmann-La Roche.
Electronic supplementary material
ESM 1
(DOC 62 kb)
Rights and permissions
About this article
Cite this article
Meulendijks, D., Beerepoot, L.V., Boot, H. et al. Trastuzumab and bevacizumab combined with docetaxel, oxaliplatin and capecitabine as first-line treatment of advanced HER2-positive gastric cancer: a multicenter phase II study. Invest New Drugs 34, 119–128 (2016). https://doi.org/10.1007/s10637-015-0309-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-015-0309-4