Summary
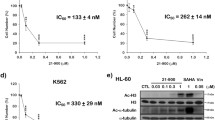
Natural products play a pivotal role in the treatment of cancer; identification of compounds such as taxanes and the vinca alkaloids were seminal landmarks in natural product drug discovery. Jerantinine A, a novel Aspidosperma alkaloid isolated from plant species Tabernaemontana corymbosa, was previously reported to possess cytotoxic activity against vincristine-resistant nasopharyngeal carcinoma cells and is therefore an ideal candidate for biological investigation. Furthermore, Tabernaemontana corymbosa, has been placed in the endangered list of threatened species by the International Union for Conservation of Nature thus making it a priority to elucidate the biological activity of this alkaloid. Herein, we report detailed biological evaluation of jerantinine A on various human-derived carcinoma cell lines. Our preliminary screens showed that significant inhibition of cell growth and colony formation accompanied time- and dose-dependent induction of apoptosis in human cancer cell lines after treatment with jerantinine A. Dose-dependent accumulations of cleaved PARP and caspase 3 further confirmed apoptosis. Profound G2/M cell cycle arrest was observed 24 h after treatment in all cell lines. Characteristics of mitotic arrest including inhibition of tubulin polymerisation, microtubule disruption, aneuploidy, and cyclin B1 down-regulation were clearly observed. The potent anti-proliferative, pro-apoptotic, and tubulin-destabilising activities of jerantinine A warrant further development of this molecule as a potential chemotherapeutic agent.









Similar content being viewed by others
References
Cragg GM, Newman DJ (2005) Biodiversity: a continuing source of novel drug leads. Pure Appl Chem 77(1):7–24. doi:10.1351/pac200577010007
Mann J (2002) Natural products in cancer chemotherapy: past, present and future. Nat Rev Cancer 2(2):143–148
Newman DJ, Cragg GM (2007) Natural products as sources of new drugs over the past 25 years. J Nat Prod 70(3):461–477
Newman DJ, Cragg GM, Sander K (2003) Natural products as sources of new drugs over the period of 1981–2002. J Nat Prod 66(7):1022–1037
Newman DJ, Cragg GM (2012) Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod 75(3):311–335. doi:10.1021/np200906s
Lam F, Bradshaw TD, Mao H, Roberts S, Pan Y, Wang S (2012) ZJU-6, a novel derivative of Erianin, shows potent anti-tubulin polymerisation and anti-angiogenic activities. Investig New Drugs 30(5):1899–1907. doi:10.1007/s10637-011-9755-9
Gascoigne KE, Taylor SS (2009) How do anti-mitotic drugs kill cancer cells? J Cell Sci 122(Pt 15):2579–2585. doi:10.1242/jcs.039719
Lobert S, Vulevic B, Correia JJ (1996) Interaction of vinca alkaloids with tubulin: a comparison of vinblastine, vincristine, and vinorelbine. Biochemistry 35:6806–6814
Amon A (1999) The spindle checkpoint. Curr Opin Cell Biol 9:69–75
Burke DJ (2000) Complexity in the spindle checkpoint. Curr Opin Cell Biol 10:26–31
Rudner AD, Murray AW (1996) The spindle assembly checkpoint. Curr Opin Cell Biol 8:773–780
Blajeski AL, Phan VA, Kottke TJ, Kaufmann SH (2002) G1 and G2 cell-cycle arrest following microtubule depolymerization in human breast cancer cells. J Clin Investig 110(1):91–99. doi:10.1172/jci200213275
Vermuelen K, Bockstaele DRV, Berneman ZN (2003) The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif 36:131–149
Ling YH, Consoli U, Tornos C, Andreeff M, Perez-Soler R (1998) Accumulation of cyclin B1, activation of cyclin B1-dependent kinase and induction of programmed cell death in human epidermoid carcinoma KB cells treated with taxol. Int J Cancer 75(6):925–932
Tu Y, Cheng S, Zhang S, Sun H, Xu Z (2013) Vincristine induces cell cycle arrest and apoptosis in SH-SY5Y human neuroblastoma cells. Int J Mol Med 31(1):113–119. doi:10.3892/ijmm.2012.1167
Lim K-H, Hiraku O, Komiyama K, Kam T-S (2008) Jerantinines A-G, cytotoxic aspidosperma alkaloids from tabernaemontana corymbosa. J Nat Prod 71:1591–1594
Bradshaw TD, Stone EL, Trapani V, Leong CO, Matthews CS, te Poele R, Stevens MF (2008) Mechanisms of acquired resistance to 2-(4-Amino-3-methylphenyl)benzothiazole in breast cancer cell lines. Breast Cancer Res Treat 110(1):57–68. doi:10.1007/s10549-007-9690-9
Nicoletti I (1991) A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods 132(2):272–279
Lee JC, Timasheff SN (1977) In vitro reconstitution of calf brain microtubules: effects of solution variables. Biochemistry 16(8):1754–1764
Shelanski M, Gaskin F, Cantor C (1973) Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A 70(3):765–768
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen R, Keating MJ, Gandhi V, Plunkett W (2005) Transcription inhibition by flavopiridol: mechanism of chronic lymphocytic leukemia cell death. Blood 106:2513–2519
Collins HM, Abdelghany MK, Messmer M, Yue B, Deeves SE, Kindle KB, Mantelingu K, Aslam A, Winkler GS, Kundu TK, Heery DM (2013) Differential effects of garcinol and curcumin on histone and p53 modifications in tumour cells. BMC Cancer 13:37. doi:10.1186/1471-2407-13-37
Lazo JS, Tamura K, Vogt A, Jung J-K, Rodriguez S, Balachandran R, Day BW, Wipf P (2000) Antimitotic actions of a novel analog of the fungal metabolite palmarumycin CP1. J Pharmacol Exp Ther 296(2):364–371
Yuan J, Yan R, Kramer A, Eckerdt F, Roller M, Kaufmann M, Strebhardt K (2004) Cyclin B1 depletion inhibits proliferation and induces apoptosis in human tumor cells. Oncogene 23(34):5843–5852. doi:10.1038/sj.onc.1207757
Rowinsky EK (1997) The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu Rev Med 48:353–374
Xiao H, Verdier-Pinard P, Fernandez-Fuentes N, Burd B, Angeletti R, Fiser A, Horwitz SB, Orr GA (2006) Insights into the mechanism of microtubule stabilization by taxol. Proc Natl Acad Sci U S A 103(27):10166–10173. doi:10.1073/pnas.0603704103
Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell. J (2000) Section 19.3: kinesin, dynein, and intracellular transport. In: Molecular cell biology. 4th edn. W. H. Freeman, New York
Cooper GM (2000) Microtubules. In: The cell: a molecular approach. 2nd edn. Sinauer Associates, Boston University
Frei R, Staedler D, Raja A, Franke R, Sasse F, Gerber-Lemaire S, Waser J (2013) Total synthesis and biological evaluation of jerantinine E. Angew Chem. doi:10.1002/anie.201305533
Gonzalez-Cid M, Cuello MT, Larripa I (1999) Comparison of the aneugenic effect of vinorelbine and vincristine in cultures human lymphocytes. Mutagenesis 14(1):63–66
Miller BM, Adler ID (1989) Suspect spindle poisons: analysis of c-mitotic effects in mouse bone marrow cells. Mutagenesis 4:208–215
Chen Q, Zhang X, Jiang Q, Clarke PR, Zhang C (2008) Cyclin B1 is localized to unattached kinetochores and contributes to efficient microtubule attachment and proper chromosome alignment during mitosis. Cell Res 18(2):268–280. doi:10.1038/cr.2008.11
Hsu MH, Liu CY, Lin CM, Chen YJ, Chen CJ, Lin YF, Huang LJ, Lee KH, Kuo SC (2012) 2-(3-methoxyphenyl)-5-methyl-1,8-naphthyridin-4(1H)-one (HKL-1) induces G2/M arrest and mitotic catastrophe in human leukemia HL-60 cells. Toxicol Appl Pharmacol 259(2):219–226. doi:10.1016/j.taap.2011.12.026
Jordan MA, Wilson L (2004) Microtubules as a target for anticancer drugs. Nat Rev Cancer 4(4):253–265. doi:10.1038/nrc1317
Hengartner M (2000) Biochemistry of apoptosis. Nature 407:770
Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S (1998) A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391:43–50
Podar K, Gouill SL, Zhang J, Opferman JT, Zorn E, Tai YT, Hideshima T, Amiot M, Chauhan D, Harousseau JL, Anderson KC (2008) A pivotal role for Mcl-1 in Bortezomib-induced apoptosis. Oncogene 27(6):721–731. doi:10.1038/sj.onc.1210679
Hu J, Dang N, Song T, Vanderkerken K (2011) Mcl-1 reduction due to caspase-dependent cleavage during endoplasmic reticulum stress-induced apoptosis. J Biol Chem 286(44):le24. doi:10.1074/jbc.L111.233502, author reply le25
Wetzler M, Segal D (2011) Omacetaxine as an anticancer therapeutic: what is old is new again. Curr Pharm Des 17(1):59–64
Chen R, Guo L, Chen Y, Jiang Y, Wierda WG, Plunkett W (2011) Homoharringtonine reduced Mcl-1 expression and induced apoptosis in chronic lymphocytic leukemia. Blood 117(1):156–164. doi:10.1182/blood-2010-01-262808
Zhu BK, Wang P, Zhang XD, Jiang CC, Chen LH, Avery-Kiejda KA, Watts R, Hersey P (2008) Activation of Jun N-terminal kinase is a mediator of vincristine-induced apoptosis of melanoma cells. Anti-Cancer Drugs 19(2):189–200. doi:10.1097/CAD.0b013e3282f3138a
Tan G, Heqing L, Jiangbo C, Ming J, Yanhong M, Xianghe L, Hong S, Li G (2002) Apoptosis induced by low-dose paclitaxel is associated with p53 upregulation in nasopharyngeal carcinoma cells. Int J Cancer 97:168–172
Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF (2001) Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol 3:339–345
Cohen GM, Sun X-M, Snowden RT, Dinsdale D, Skilleter DN (1992) Key morphological features of apoptosis may occur in the absence of internucleosomal DNA fragmentation. Biochem J 286:331–334
Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L (1996) Mitotic block induced in HeLa cells by low concentrations of paclitaxel (taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res 56(4):816–825
Eitaki M, Yamamori T, Meike S, Yasui H, Inami O (2012) Vincristine enhances amoeboid-like motility via GEF-H1/RhoA/ROCK/Myosin light chain signaling in MKN45 cells. BMC Cancer 12(469):1–12
Fadoka VA, Bratton DL, Frasch SC, Warner ML, Henson PM (1998) The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ 5(7):551–562
Acknowledgments
Authors would like to thank Rachel Anderson and Dr. Hilary Collins. T.S. Kam and K.H. Lim thank MOHE, Malaysia (HIR-005) for financial support.
Ethical standards declaration
The authors declare that the experiments comply with the current laws of the country in which they were performed.
Conflict of interest statement
Authors have no conflicts of interest to declare.
Signed:

On behalf of all authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raja, V.J., Lim, KH., Leong, CO. et al. Novel antitumour indole alkaloid, Jerantinine A, evokes potent G2/M cell cycle arrest targeting microtubules. Invest New Drugs 32, 838–850 (2014). https://doi.org/10.1007/s10637-014-0126-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-014-0126-1