Summary
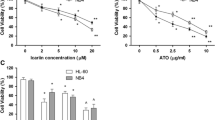
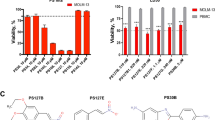
Arsenic trioxide (ATO) is an inorganic arsenic derivative that is highly effective against PML-RARα-positive leukemia but much less against other hematological malignancies. We synthesized an organic arsenic derivative (OAD), S-dimethylarsino-thiosuccinic acid (MER1), which offers a superior toxicity profile and comparable in vitro activity relative to ATO. In Swiss Webster mice, maximally-tolerated cumulative dose of MER1 when given IV for 5 days was 100 mg/kg/d. We demonstrated that MER1 induced apoptosis and dose- and time-dependent inhibition of survival and growth in a panel of myeloid leukemia cell lines. Unlike ATO, this activity was independent of PML-RARα status and was not associated with induction of myeloid maturation. In NB4 and HL60 cells, MER1 and ATO induced caspase activation and dissipation of mitochondrial transmembrane potential. At the same time, MER1 induced generation of reactive oxygen species (ROS) and cell cycle arrest in G2/M phase and proved to be more potent than ATO at inducing apoptosis. ROS generation and intracellular glutathione levels were key modulators of MER1-induced cytotoxicity as evidenced by abrogation of apoptosis in myeloid leukemia cell lines pretreated with the disulfide bond-reducing agent dithiothreitol or the radical scavenger N-acetyl-L-cysteine. Collectively, these data indicate that MER1 induces apoptosis in PML-RARα-positive and -negative myeloid leukemia cells by enhancing oxidative stress. This agent, therefore, combines low in vivo toxicity with formidable in vitro pro-apoptotic ROS-mediated activity, and may represent a novel OAD suitable for clinical development against a variety of hematological malignancies.






Similar content being viewed by others
References
de The H, Chomienne C, Lanotte M, Degos L, Dejean A (1990) The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature 347(6293):558–561. doi:10.1038/347558a0
Borrow J, Goddard AD, Sheer D, Solomon E (1990) Molecular analysis of acute promyelocytic leukemia breakpoint cluster region on chromosome 17. Science 249(4976):1577–1580. doi:10.1126/science.2218500
Shao W, Fanelli M, Ferrara FF, Riccioni R, Rosenauer A, Davison K, Lamph WW, Waxman S, Pelicci PG, Lo Coco F et al (1998) Arsenic trioxide as an inducer of apoptosis and loss of PML/RAR alpha protein in acute promyelocytic leukemia cells. J Natl Cancer Inst 90(2):124–133. doi:10.1093/jnci/90.2.124
Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, Jin XL, Tang W, Li XS, Xong SM et al (1996) In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML. Blood 88(3):1052–1061
Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, Corso D, DeBlasio A, Gabrilove J, Scheinberg DA et al (1998) Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med 339(19):1341–1348. doi:10.1056/NEJM199811053391901
Miller WH Jr, Schipper HM, Lee JS, Singer J, Waxman S (2002) Mechanisms of action of arsenic trioxide. Cancer Res 62(14):3893–3903
Chou WC, Hawkins AL, Barrett JF, Griffin CA, Dang CV (2001) Arsenic inhibition of telomerase transcription leads to genetic instability. J Clin Invest 108(10):1541–1547
Chen GQ, Zhou L, Styblo M, Walton F, Jing Y, Weinberg R, Chen Z, Waxman S (2003) Methylated metabolites of arsenic trioxide are more potent than arsenic trioxide as apoptotic but not differentiation inducers in leukemia and lymphoma cells. Cancer Res 63(8):1853–1859
Duzkale H, Jilani I, Orsolic N, Zingaro RA, Golemovic M, Giles FJ, Kantarjian H, Albitar M, Freireich EJ, Verstovsek S (2003) In vitro activity of dimethylarsinic acid against human leukemia and multiple myeloma cell lines. Cancer Chemother Pharmacol 51(5):427–432
Verstovsek S, Giles F, Quintas-Cardama A, Perez N, Ravandi-Kashani F, Beran M, Freireich E, Kantarjian H (2006) Arsenic derivatives in hematologic malignancies: a role beyond acute promyelocytic leukemia? Hematol Oncol 24(4):181–188. doi:10.1002/hon.787
Verstovsek S, Duzkale, H, Orsolic, N et al (2002) S-dimethylarsino-thiosuccinic acid, an organic arsenic derivative, is comparable in in vitro antileukemic activity to arsenic trioxide but possesses significantly less toxicity. Blood 100(11):(abstr 4614)
Yi J, Gao F, Shi G, Li H, Wang Z, Shi X, Tang X (2002) The inherent cellular level of reactive oxygen species: one of the mechanisms determining apoptotic susceptibility of leukemic cells to arsenic trioxide. Apoptosis 7(3):209–215. doi:10.1023/A:1015331229263
Rojkind M, Dominguez-Rosales JA, Nieto N, Greenwel P (2002) Role of hydrogen peroxide and oxidative stress in healing responses. Cell Mol Life Sci 59(11):1872–1891. doi:10.1007/PL00012511
Jing Y, Dai J, Chalmers-Redman RM, Tatton WG, Waxman S (1999) Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood 94(6):2102–2111
Grignani F, Ferrucci PF, Testa U, Talamo G, Fagioli M, Alcalay M, Mencarelli A, Peschle C, Nicoletti I et al (1993) The acute promyelocytic leukemia-specific PML-RAR alpha fusion protein inhibits differentiation and promotes survival of myeloid precursor cells. Cell 74(3):423–431. doi:10.1016/0092-8674(93)80044-F
Pan J, Quintas-Cardama A, Kantarjian HM, Akin C, Manshouri T, Lamb P, Cortes JE, Tefferi A, Giles FJ, Verstovsek S (2007) EXEL-0862, a novel tyrosine kinase inhibitor, induces apoptosis in vitro and ex vivo in human mast cells expressing the KIT D816V mutation. Blood 109(1):315–322. doi:10.1182/blood-2006-04-013805
Takuma T, Takeda K, Konno K (1987) Synergism of tumor necrosis factor and interferon-gamma in induction of differentiation of human myeloblastic leukemic ML-1 cells. Biochem Biophys Res Commun 145(1):514–521. doi:10.1016/0006-291X(87)91351-9
Hultdin M, Gronlund E, Norrback K, Eriksson-Lindstrom E, Just T, Roos G (1998) Telomere analysis by fluorescence in situ hybridization and flow cytometry. Nucleic Acids Res 26(16):3651–3656. doi:10.1093/nar/26.16.3651
Rego EM, He LZ, Warrell RP Jr, Wang ZG, Pandolfi PP (2000) Retinoic acid (RA) and As2O3 treatment in transgenic models of acute promyelocytic leukemia (APL) unravel the distinct nature of the leukemogenic process induced by the PML-RARalpha and PLZF-RARalpha oncoproteins. Proc Natl Acad Sci USA 97(18):10173–10178. doi:10.1073/pnas.180290497
Zhang W, Ohnishi K, Shigeno K, Fujisawa S, Naito K, Nakamura S, Takeshita K, Takeshita A, Ohno R (1998) The induction of apoptosis and cell cycle arrest by arsenic trioxide in lymphoid neoplasms. Leukemia 12(9):1383–1391. doi:10.1038/sj.leu.2401112
Park JW, Choi YJ, Jang MA, Baek SH, Lim JH, Passaniti T, Kwon TK (2001) Arsenic trioxide induces G2/M growth arrest and apoptosis after caspase-3 activation and bcl-2 phosphorylation in promonocytic U937 cells. Biochem Biophys Res Commun 286(4):726–734. doi:10.1006/bbrc.2001.5416
Griffith OW, Meister A (1979) Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem 254(16):7558–7560
Boldin MP, Goncharov TM, Goltsev YV, Wallach D (1996) Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell 85(6):803–815. doi:10.1016/S0092-8674(00) 81265-9
Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281(5381):1309–1312. doi:10.1126/science.281.5381.1309
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91(4):479–489. doi:10.1016/S0092-8674(00)80434-1
Qian Y, Castranova V, Shi X (2003) New perspectives in arsenic-induced cell signal transduction. J Inorg Biochem 96(2–3):271–278. doi:10.1016/S0162-0134(03)00235-6
Halliwell B (1999) Oxygen and nitrogen are pro-carcinogens. Damage to DNA by reactive oxygen, chlorine and nitrogen species: Measurement, mechanism and the effects of nutrition. Mutat Res 443(1–2):37–52
Davison K, Cote S, Mader S, Miller WH (2003) Glutathione depletion overcomes resistance to arsenic trioxide in arsenic-resistant cell lines. Leukemia 17(5):931–940. doi:10.1038/sj.leu.2402876
Gao F, Yi J, Yuan JQ, Shi GY, Tang XM (2004) The cell cycle related apoptotic susceptibility to arsenic trioxide is associated with the level of reactive oxygen species. Cell Res 14(1):81–85. doi:10.1038/sj.cr.7290206
Acknowledgement
Robert A. Welch Foundation sponsored work done by RAZ.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Suppl. Fig. 1
NB4 cells were treated for 24 h with ATO (lanes 1–4) or MER1 (lanes 5–8) at 0.5 μM, 1, 5 μM, and 10 μM, respectively. Degradation of Bcl-2 protein was observed by immunoprecipitation after ATO (but not MER1) treatment. Bcl-2 expression in untreated NB4 cells is presented in lane 9. (PPT 238 kb)
Suppl. Fig. 2
HL60 cells were treated with MER1 (5 μM) alone or in combination with NAC (10 mM) for 8 h, 14 h, or 24 h. The percentage of cells in G2/M phase were detected by flow cytometry. The appropriate controls with only 10 mM NAC added were prepared as well. They did not differ from displayed non-treated controls. (PPT 148 kb)
Suppl. Fig. 3
MER1 treatment is not associated with telomere shortening. Contour plots showing PNA probe fluorescence versus DNA content in: (A) NB4 and CEM-1301 cells; (B) NB4 cells cultured in the presence of ATO 1 μM and CEM-1301 cells; (C) NB4 cells cultured in the presence of MER1 1 μM and CEM-1301 cells. (PPT 85 kb)
Rights and permissions
About this article
Cite this article
Golemovic, M., Quintás-Cardama, A., Manshouri, T. et al. MER1, a novel organic arsenic derivative, has potent PML-RARα- independent cytotoxic activity against leukemia cells. Invest New Drugs 28, 402–412 (2010). https://doi.org/10.1007/s10637-009-9267-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-009-9267-z