Abstract
Background/Aims
A high-fat diet (HFD) can cause intestinal inflammation and alter the gut microbiota; probiotics, however, are known to have anti-inflammatory effects. This study aimed to investigate the response of rat colon to HFD and the effect of Clostridium butyricum on HFD-induced intestinal inflammation and production of short-chain fatty acids (SCFAs) according to sex.
Methods
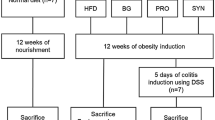
Male and female 6-week-old Fischer-344 rats were fed a chow diet or HFD for 8 weeks, and Biovita or three different concentrations of C. butyricum were orally gavaged. The levels of tight junction proteins (TJPs), inflammatory markers in the ascending colonic mucosa, and bile acids (BAs) and SCFAs in stool were measured.
Results
HFD significantly increased the histological inflammation scores and fat proportions. Fecal BA levels were higher in the HFD group than in the control group, with a more prominent increase in deoxycholic acid/cholic acid after probiotics administration in females; however, no statistically significant differences were observed. TJPs showed an opposite response to HFD depending on sex, and tended to increase and decrease after HFD in males and females, respectively. The HFD-reduced TJPs were recovered by probiotics, with some statistical significance in females. HFD-decreased butyric acid in stools appeared to be recovered by probiotics in males, but not in females. The expression of inflammatory markers (TNF-α) was increased by HFD in males and decreased with medium-concentration probiotic supplementation. The opposite was observed in females. MPO was increased by HFD in both sexes and decreased by probiotic supplementation.
Conclusions
The probiotic C. butyricum improved indicators of HFD-induced colonic inflammation such as levels of inflammatory markers and increased the production of SCFAs and the expression of TJPs. These effects tended to be more pronounced in male rats, showing sex difference.







Similar content being viewed by others
Abbreviations
- HFD:
-
High-fat diet
- C. butyricum :
-
Clostridium butyricum
- BA:
-
Bile acid
- TJP:
-
Tight function protein
- MPO:
-
Myeloperoxidase
- IL:
-
Interleukin
- TNF:
-
Tumor necrosis factor
- SCFA:
-
Short-chain fatty acid
- SPF:
-
Specific-pathogen-free
- PBS:
-
Phosphate-buffered saline
- CFU:
-
Colony-forming unit
- ELISA:
-
Enzyme-linked immunosorbent assay
- RT-qPCR:
-
Real-time quantitative polymerase chain reaction
- H&E:
-
Hematoxylin and eosin
- CA:
-
Cholic acid
- CDCA:
-
Chenodeoxycholic acid
- DCA:
-
Deoxycholic acid
- IBS:
-
Irritable bowel syndrome
References
Kim SE, Paik HY, Yoon H et al. Sex- and gender-specific disparities in colorectal cancer risk. World J Gastroenterol. 2015;21:5167–5175.
Lee SM, Kim N, Yoon H et al. Compositional and functional changes in the gut microbiota in irritable bowel syndrome patients. Gut Liver. 2021;15:253–261.
Bischoff SC. Microbiota and aging. Curr Opin Clin Nutr Metab Care. 2016;19:26–30.
Mueller S, Saunier K, Hanisch C et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol. 2006;72:1027–1033.
Claesson MJ, Jeffery IB, Conde S et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184.
Bolnick DI, Snowberg LK, Hirsch PE et al. Individual diet has sex-dependent effects on vertebrate gut microbiota. Nat Commun. 2014;5:4500.
Hildebrandt MA, Hoffmann C, Sherrill-Mix SA et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;37:1716-1724.e1-2.
Ding S, Chi MM, Scull BP et al. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS ONE. 2010;5:e12191.
Lee SM, Kim N, Yoon H et al. Microbial changes and host response in F344 rat colon depending on sex and age following a high-fat diet. Front Microbiol. 2018;9:2236.
Lee BJ, Bak YT. Irritable bowel syndrome, gut microbiota and probiotics. J Neurogastroenterol Motil. 2011;17:252–266.
Shang H, Sun J, Chen YQ. Clostridium butyricum CGMCC0313.1 modulates lipid profile, insulin resistance and colon homeostasis in obese mice. PLoS ONE. 2016;11:e0154373.
Kim MC. Utility and importance of blending of BIOVITA 3 bacterial species in intestinal health. Yakhak Hoeji. 2021;65:258–263.
Lefebvre P, Cariou B, Lien F et al. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–191.
Fiorucci S, Distrutti E. Bile acid-activated receptors, intestinal microbiota, and the treatment of metabolic disorders. Trends Mol Med. 2015;21:702–714.
Garcia-Hernandez V, Quiros M, Nusrat A. Intestinal epithelial claudins: expression and regulation in homeostasis and inflammation. Ann N Y Acad Sci. 2017;1397:66–79.
Jo HJ, Kim N, Nam RH et al. Fat deposition in the tunica muscularis and decrease of interstitial cells of Cajal and nNOS-positive neuronal cells in the aged rat colon. Am J Physiol Gastrointest Liver Physiol. 2014;306:G659-669.
Liu J, Sun J, Wang F et al. Neuroprotective effects of Clostridium butyricum against vascular dementia in mice via metabolic butyrate. Biomed Res Int. 2015;2015:412946.
Jia L, Li D, Feng N et al. Anti-diabetic effects of Clostridium butyricum CGMCC0313.1 through promoting the growth of gut butyrate-producing bacteria in type 2 diabetic mice. Sci Rep. 2017;7:7046.
Katakura K, Lee J, Rachmilewitz D et al. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest. 2005;115:695–702.
Choi SI, Son JH, Kim N et al. Changes in cecal microbiota and short-chain fatty acid during lifespan of the rat. J Neurogastroenterol Motil. 2021;27:134–146.
Kim KA, Gu W, Lee IA et al. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE. 2012;7:e47713.
Okada Y, Tsuzuki Y, Sato H et al. Trans fatty acids exacerbate dextran sodium sulphate-induced colitis by promoting the up-regulation of macrophage-derived proinflammatory cytokines involved in T helper 17 cell polarization. Clin Exp Immunol. 2013;174:459–471.
Guigoz Y, Dore J, Schiffrin EJ. The inflammatory status of old age can be nurtured from the intestinal environment. Curr Opin Clin Nutr Metab Care. 2008;11:13–20.
Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323.
Lee SM, Kim N, Park JH et al. Comparative analysis of ileal and cecal microbiota in aged rats. J Cancer Prev. 2018;23:70–76.
Lee JY, Kim N, Kim YS et al. Repeated water avoidance stress alters mucosal mast cell counts, interleukin-1βâ levels with sex difference in distal colon of Wistar rats. J Neurogastroenterol Motil. 2016;22:694–704.
Lee JY, Kim N, Nam RH et al. Probiotics reduce repeated water avoidance stress-induced colonic microinflammation in Wistar rats in a sex-specific manner. PLoS ONE. 2017;12:e0188992.
Son HJ, Kim N, Song CH et al. Sex-related alterations of gut microbiota in the C57BL/6 mouse model of inflammatory bowel disease. J Cancer Prev. 2019;24:173–182.
Choi SI, Kim N, Nam RH et al. Fecal microbial enterotypes differentially respond to a high-fat diet based on sex in Fischer-344 rats. J Cancer Prev. 2021;26:277–288.
Choi SI, Kim N, Lee SM et al. Rat intestinal acetic acid and butyric acid and effects of age, sex, and high-fat diet on the intestinal levels in rats. J Cancer Prev. 2019;24:20–25.
Yoon K, Kim N. Roles of sex hormones and gender in the gut microbiota. J Neurogastroenterol Motil. 2021;27:314–325.
Menon R, Watson SE, Thomas LN et al. Diet complexity and estrogen receptor β status affect the composition of the murine intestinal microbiota. Appl Environ Microbiol. 2013;79:5763–5773.
Kwa M, Plottel CS, Blaser MJ, Adams S. The intestinal microbiome and estrogen receptor-positive female breast cancer. J Natl Cancer Inst. 2016;108:djw029.
Kim N. Sex difference of gut microbiota. Kim N, eds. Sex/gender-specific medicine in the gastrointestinal diseases pp 363–377. S Singapore. 2022.
Kaliannan K, Robertson RC, Murphy K et al. Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome. 2018;6:205.
Flores R, Shi J, Fuhrman B et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J Transl Med. 2012;10:253.
Kim YS, Kim N. Sex-gender differences in irritable bowel syndrome. J Neurogastroenterol Motil. 2018;24:544–558.
Labianca R, Beretta GD, Kildani B et al. Colon cancer. Crit Rev Oncol Hematol. 2010;74:106–133.
Vowinkel T, Kalogeris TJ, Mori M et al. Impact of dextran sulfate sodium load on the severity of inflammation in experimental colitis. Dig Dis Sci. 2004;49:556–564.
Roncucci L, Mora E, Mariani F et al. Myeloperoxidase-positive cell infiltration in colorectal carcinogenesis as indicator of colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17:2291–2297.
Rivière A, Selak M, Lantin D et al. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol. 2016;7:979.
Guo P, Zhang K, Ma X, He P. Clostridium species as probiotics: potentials and challenges. J Anim Sci Biotechnol. 2020;11:24.
Ou J, Carbonero F, Zoetendal EG et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013;98:111–120.
Wu X, Wu Y, He L et al. Effects of the intestinal microbial metabolite butyrate on the development of colorectal cancer. J Cancer. 2018;9:2510–2517.
Yoon K, Kim N. The effect of microbiota on colon carcinogenesis. J Cancer Prev. 2018;23:117–125.
Chen J, Vitetta L. Inflammation-modulating effect of butyrate in the prevention of colon cancer by dietary fiber. Clin Colorectal Cancer. 2018;17:e541-544.
de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013;17:657–669.
Swann JR, Want EJ, Geier FM et al. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci USA 2011;108:4523–4530.
Begley M, Gahan CG, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005;29:625–651.
Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259.
Nakade Y, Kitano R, Sakamoto K et al. Characteristics of bile acid composition in high fat diet-induced nonalcoholic fatty liver disease in obese diabetic rats. PLoS ONE. 2021;16:e0247303.
Yang ZH, Liu F, Zhu XR et al. Altered profiles of fecal bile acids correlate with gut microbiota and inflammatory responses in patients with ulcerative colitis. World J Gastroenterol. 2021;27:3609–3629.
Midzak A, Papadopoulos V. Binding domain-driven intracellular trafficking of sterols for synthesis of steroid hormones, bile acids and oxysterols. Traffic. 2014;15:895–914.
Lee JY, Kim N, Park JH et al. Expression of neurotrophic factors, tight junction proteins, and cytokines according to the irritable bowel syndrome subtype and sex. J Neurogastroenterol Motil. 2020;26:106–116.
Varghese M, Griffin C, McKernan K et al. Sex differences in inflammatory responses to adipose tissue lipolysis in diet-induced obesity. Endocrinology. 2019 Feb;1:293–312.
Ter Horst R, van den Munckhof ICL, Schraa K et al. Sex-specific regulation of inflammation and metabolic syndrome in obesity. Arterioscler Thromb Vasc Biol. 2020;40:1787–1800.
Sung MK, Yeon JY, Park SY et al. Obesity-induced metabolic stresses in breast and colon cancer. Ann N Y Acad Sci. 2011;1229:61–68.
Heo JW, Kim SE, Sung MK. Sex differences in the incidence of obesity-related gastrointestinal cancer. Int J Mol Sci. 2021;22:1253.
Funding
This work was supported by Grant No. 06-2020-0317 from the Seoul National University Bundang Hospital Research Fund. In addition, this work was supported by Ildong Pharmaceutical, Co., Ltd.
Author information
Authors and Affiliations
Contributions
Conceptualization and generator: NK. Data curation: SIC, NK. Formal analysis: YC, SIC. Funding acquisition: NK. Investigation: SIC, RHN, JYJ, NK. Methodology: SIC, RHN, JYJ, NK. Project administration: NK, Y-JS. Resources: HM, Y-RK. Supervision: NK, HYN, Y-JS. Writing—original draft: YC, SIC. Writing—review and editing: NK, CMS, DHL.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest relevant to this article to disclose.
Ethical approval
This study was carried out in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of South Korea. The protocol was approved by the Institutional Animal Care and Use Committee of Seoul National University Bundang Hospital (Permission No. BA1506-178/027-01).
Informed consent
This study is exempt from consent, since it is an animal experiment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Choi, Y., Choi, S.I., Kim, N. et al. Effect of Clostridium butyricum on High-Fat Diet-Induced Intestinal Inflammation and Production of Short-Chain Fatty Acids. Dig Dis Sci 68, 2427–2440 (2023). https://doi.org/10.1007/s10620-023-07835-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-07835-2