Abstract
Background
Proton pump inhibitor (PPI) use has increased over the last decades and has been associated with multiple adverse events and potentially even overall survival.
Aims
We aimed to investigate the association between proton pump inhibitor maintenance use and all-cause and cause-specific mortality, addressing confounding by indication and duration of use.
Methods
This Swedish population-based cohort study included all adult (N = 935,236) PPI and histamine-2 receptor antagonist maintenance users (≥ 180 days use) during 2005–2014. Standardised mortality ratios (SMRs) and 95% confidence intervals were calculated for all-cause and cause-specific mortality comparing the risk among PPI/H2RA users to that of the Swedish background population, stratified by age, sex, calendar period, indication and duration of use. Multivariable Poisson regression models were used to compare PPI use to H2RA use, expressed as incidence rate ratios and 95% confidence intervals.
Results
PPI and histamine-2 receptor antagonist use were associated with an increased risk of all-cause mortality (SMR = 1.35; 1.34–1.36; SMR = 1.31; 1.27–1.36, respectively). The highest SMRs were found in the youngest age groups. In direct comparison, PPI use showed a higher mortality risk than histamine-2 receptor antagonist use (incidence rate ratios = 1.42; 1.38–1.46). PPIs were related to increased cancer (SMR = 1.21; 1.20–1.22), and cardiovascular mortality (SMR = 1.36; 1.35–1.37). Increased SMRs were observed for most indications. Longer duration of use was associated with a higher mortality among PPI users but not among histamine-2 receptor antagonist users.
Conclusion
Maintenance PPI use was associated with an increased risk of all-cause and cause-specific mortality, and the risk increased with prolonged duration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proton Pump Inhibitors (PPIs) are one of the most frequently prescribed drugs worldwide used by up to 30% of adults [1,2,3,4,5,6,7]. They are commonly prescribed for the suppression of gastric acid production in the management of gastrointestinal acid-related disorders which include gastroesophageal reflux disease, peptic ulcer disease, and dyspepsia [1, 2, 5, 8]. They are available over the counter in many countries, including Sweden. Swedish clinical guidelines recommend short-term treatment for 8–12 weeks for most indications [1, 6]. Yet they can also be used as maintenance treatment for symptom control in individuals with chronic conditions such as refractory reflux disease (persistent symptoms), hypersecretory disorders (e.g. Barrett oesophagus, Zollinger–Ellison syndrome) and for gastroprotection among aspirin or other nonsteroidal anti-inflammatory drug (NSAIDs) users [5, 8,9,10,11,12].
The maintenance use of PPIs has increased remarkably during recent years, and some studies estimate long-term acid suppression accounts for up to 50–66% of the total use [9, 10]. Worrisomely, a significant proportion of PPI users lack a clear indication for the treatment, and inappropriate prescriptions are observed in 25–70% of cases [1, 2, 10, 13, 14]. Evidence suggests that PPIs are rarely de-prescribed, sometimes continued for long-time periods without proper reassessment resulting in an increase in long-term users over the years [1, 8, 15, 16].
Concern over the safety of long-term use of PPIs has grown, due to the observed associations between PPI use and serious adverse events such as chronic kidney disease, cardiovascular disease, and cancers of the gastrointestinal tract, some of which are also associated with an increased risk of mortality [4, 17,18,19,20,21,22,23]. Furthermore, some recent studies describe increased all-cause and cause-specific mortality in PPI users [3, 17, 22, 23]. Two large observational studies from the US (Department of Veteran affairs-VA study) reported 17–25% increased mortality risk among PPI users compared to Histamine-2 receptor antagonists (H2RAs) users [3, 22, 24], while a UK study also found an association between PPI use and all-cause and cause-specific mortality [23]. However, these studies were limited by residual confounding, confounding by indication, and limited generalisability [3, 4, 22,23,24,25]. In contrast, a recent 3-year randomised controlled trial did not confirm the findings in the above studies, yet it had limited power [24, 26]. Data from large, nationwide population-based studies that span a reasonably long duration of follow-up on PPI use associated mortality are scarce, and the association remains understudied.
We, therefore, sought to examine the association between PPI maintenance use and all-cause and cause-specific mortality in a Swedish population-based cohort previously used to assess the association with various cancer types.
Methods
Study Design
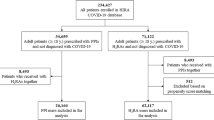
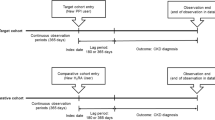
The study cohort included all adult (≥ 20 years) individuals who received prescribed maintenance treatment of PPIs or H2RAs between July 1, 2005 (start of Prescribed Drug Registry), and December 31, 2014, in Sweden. The source cohort is described in detail elsewhere [8, 25, 27, 28]. The index date was the first dispensed prescription date of a PPI (or H2RA), and participants were followed up until the occurrence of death or the end of study, whichever occurred first. The data were linked by the National Board of Health and Welfare using the unique Swedish personal identity number assigned to all Swedish residents. The study was approved by the Stockholm Regional Ethical Review Board (2014/1291-31/4), without the need for informed consent due to the registry-based nature of the study.
Exposure
Maintenance use of PPIs (or H2RAs) was defined as ≥ 180 days of accumulated use during the study period identified from the Prescribed Drug Registry (outpatient care drug use based on ATC codes, from July 2005), based on the Anatomical Therapeutic Chemical classification (ATC) system codes (PPIs: A02BC; H2RAs: A02BA). H2RAs were assessed as active comparator, because of the similar indications as PPIs. The total cumulative dosage was estimated by accumulating the Defined Daily Dosage (DDD) per package (DDDp), which considers the potency and prescribed quantities of the drug. The DDD estimates the assumed average maintenance dose per day for a drug used for its main indication in adults [29].
The participants were divided into two exposure groups (mutually exclusive): (1) PPI users (≥ 180 days PPI use and < 180 days H2RA use); (2) H2RA users (≥ 180 days H2RA use and < 180 days PPI use), serving as an active comparator control. Participants who were classified as maintenance users of both PPIs and H2RAs (≥ 180 days use of both PPIs and H2RAs; N = 30,042) were excluded from the study. To avoid reverse causation (confounding by pre-existing disease/protopathic bias) and ensure the temporal direction between drug use and diseases that lead to mortality, participants who died within a year after first exposure (n = 41,804), were excluded from the study as shown in Fig. 1. Additionally, we applied a lag time of 365 days from first dispensed prescription date to ensure exposure as per our definition.
Outcome
All-cause and cause-specific (cancer, cardiovascular and all-other causes of death) mortality were ascertained from the Swedish Causes of Death Registry (for the observed deaths), based on ICD-10 codes (Supplementary Table 1). The cause-specific mortality groups were selected and categorised based on the most common causes of death in Sweden [30]. Subgroup analyses were performed for gastrointestinal cancer (stomach; oesophageal; liver, gallbladder and bile ducts; colorectal; pancreatic and other gastrointestinal) and non-gastrointestinal cancer deaths separately. Information on the expected deaths and total person-years in the background population was retrieved from the National Board of Health and Welfare Death Registry (applying ICD-10 codes) and Statistics Sweden, respectively [31,32,33,34]. Data on cause-specific mortality were not available for deaths occurring during 2014 (as the data were retrieved early 2015).
Covariates
Standardisation occurred by age at first prescription (categorised as 20–39, 40–49, 50–59, 60–69 and ≥ 70 years), sex and calendar period. Potential confounding by indication was evaluated through subgroup analyses for the most common, non-exclusive indications for acid-suppressive treatment based on the National Patient Registry (recording all inpatient care since 1987 and specialised outpatient care since 2001): gastroesophageal reflux disease; peptic ulcers; gastroduodenitis; dyspepsia and Helicobacter pylori infection/eradication (see Supplementary Table 1 for ICD-10 codes). Maintenance use (≥ 180 days use) of aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) were identified from the Prescribed Drug Registry. Less-common indications (recorded in ≤ 5% of the study population), which include Barrett oesophagus and Zollinger–Ellison syndrome, were not assessed separately.
Statistical Analysis
Standardised mortality ratios (SMRs) with 95% confidence intervals (CIs) for all-cause and cause-specific mortality were considered as relative risks and calculated by dividing the observed mortality in the exposed cohort by the expected mortality among the entire Swedish background population, stratified by age at first prescription, sex and calendar period (categorised as 2006–2008, 2009–2011 and 2012–2014) [35]. Standardisation was performed according to Breslow and Day`s method [35].
Claytons algorithm for exact allocation of person-years was used to calculate follow-up time from first dispensed prescription date (plus 365 days) until death or end of the study period (December 2014 for all-cause mortality and December 2013 for cause-specific mortality), whichever occurred first. The chi-squared test for trend in SMRs was used to test for a linear trend between age categories and the SMRs (α = 0.05).
Furthermore, we employed an active comparator design to directly compare the mortality risks between PPI and H2RA users, and additionally assess confounding by indication, using multivariable Poisson regression models, adjusted for age, sex and the indications mentioned above. Risk was presented as adjusted incidence rate ratios (aIRR) with 95% confidence intervals. We performed Likelihood-ratio tests to test each individual predictor variable in the full multivariable model for significance.
The effect of treatment duration was assessed by evaluating the relative risk of death in relation to time since first dispensed PPI (and H2RA) prescription (categorised as 1–2.9 years, 3–4.9 years, and ≥ 5 years). All analyses were performed using STATA 14.2 (StataCorp, Texas, USA) and Microsoft Excel 14.4.0 (Microsoft Corporation).
Results
Participants
Overall, 935,236 maintenance users of acid suppression drugs were included in this study (PPIs, N = 916,823; H2RAs, N = 18,413; Table 1). There were more women (58.5%) than men (41.5%) among PPI users. The PPI users were on average younger than the H2RA users (59 years, SD ± 16.4; 62 years, SD ± 16.2; respectively). Of those with recorded indications (84.8%), the most common indications were NSAIDs use (50.0%) and aspirin use (34.4%), for PPI users; a similar distribution was observed for H2RA users (NSAIDs use-50.2%; Aspirin use-32.8%). PPI users were more likely to have multiple indications compared to H2RA users.
Median duration of follow-up was 5.9 years (interquartile range 2.9–8.0). Overall mortality was slightly lower among PPI users (17.4%) in comparison to H2RA users (22.0%) (p < 0.001). Cardiovascular diseases were the most common cause of death in both PPI (6.2%) and H2RA (9.3%) users. Similar distributions were observed for cancer-related mortality among the PPI (3.0%) and H2RA (3.0%) users.
Risk of All-Cause Mortality by Age and Sex (PPI and H2RA Users)
Compared to the Swedish background population, all-cause mortality was higher among PPI users, (SMR = 1.35; 95% CI 1.34–1.36, Table 2), with higher SMRs observed in men (SMR = 1.43; 95% CI 1.41–1.44) than women (SMR = 1.30; 95% CI 1.29–1.31). All-cause mortality was higher in the younger age groups in PPI users and decreased with increasing age, with the highest SMRs observed in the 20–39 years age group (SMR = 5.33; 95% CI 5.00–5.67) (Table 2).
Similarly, for H2RA users, the SMRs were elevated (SMR = 1.31; 95% CI 1.27–1.36), yet, in contrast to PPI users, the SMRs were higher in women (SMR = 1.34; 95% CI 1.29–1.39) than men (SMR = 1.22; 95% CI 1.22–1.34). Similar age patterns were seen in the H2RA users as observed in the PPI users, with the highest SMRs in the 20–39 years age group (SMR = 6.63; 95% CI 4.10–10.13). The score for trend p values (p < 0.001, chi-square test), were statistically significant indicating a true age-dependency effect in the PPI use and mortality risk relationship, with higher SMRs among younger age groups.
Risk of All-Cause Mortality by Indication of Use
The SMRs for all-cause mortality were increased in both PPI and H2RA users for all indication groups except H. pylori infection/eradication (SMR = 0.95; 95% CI 0.76–1.18), in H2RA users. The highest SMRs for all-cause mortality were observed among participants with peptic ulcer disease (PPI users SMR = 1.98; 95% CI 1.96–2.01; and H2RA users SMR = 2.05; 95% CI 1.87–2.25; Table 2). The SMR for PPI users without recorded indications was elevated (SMR = 1.12; 95% CI 1.10–1.14).
Risk of Cause-Specific Mortality in PPI Users
Increased mortality risks in PPI users were observed for cardiovascular (SMR = 1.36; 95% CI 1.35–1.37) and cancer-specific mortality (SMR = 1.21; 95% CI 1.20–1.22) (Fig. 2). Cause-specific mortality risks also decreased with increasing age, with the highest SMRs observed in the younger age groups. Cause-specific results could not be calculated for H2RA because of lack of power. The proportion of non-gastrointestinal and gastrointestinal causes of death for all groups is presented in Table 3; showing a larger proportion of gastrointestinal deaths among PPI users compared to the background population, both in men (38.9% versus 30.2%) and women (35.0% vs 30.8%). Elevated SMRs for gastrointestinal (SMR = 1.45, 95% CI 1.42–1.48) and non-gastrointestinal cancer (SMR = 1.11, 95% CI 1.09–1.13) were observed in PPI users compared to the background population (Table 4). SMRs were elevated for all gastrointestinal cancer subtypes, particularly gastric (SMR = 1.91, 1.81–2.01) and oesophageal cancer (SMR = 2.09, 1.95–2.24) (Table 4).
Risk of All-Cause Mortality in PPI Vs H2RA Users (Active Comparator Design)
In the direct comparison using multivariable Poisson regression, the risk of all-cause mortality was higher among PPI users than H2RA users (aIRR = 1.42; 95% CI 1.38–1.46) (Table 5). The aIRRs were higher among men (aIRR = 1.56; 95% CI 1.48–1.64) than women (aIRR = 1.32; 95% CI 1.27–1.38), and the age stratified aIRRs also showed an age-dependent effect, with the highest IRRs found in the 50–59 years age group (aIRR = 1.60; 95% CI 1.41–1.83). The mortality risks were increased in all the indication subgroups except in gastro-oesophageal reflux (aIRR = 0.93; 95% CI 0.84–1.02).
Duration of Use
The association between PPI use and the risk of all-cause mortality appeared to be dose dependant, with the highest risk for mortality observed among individuals exposed to PPIs > 5 years (SMR = 1.29; 95% CI 1.28–1.30). In contrast, the high SMRs within the first 3 years of H2RA use were attenuated with longer follow-up and became close to unity by > 5 years of use as shown in Fig. 3.
Discussion
In this large population-based study, maintenance use of gastric acid suppressors was associated with increased relative risks of all-cause and cause-specific mortality. The risk of all-cause mortality was elevated in both PPI and H2RA maintenance users, particularly among the younger age groups. The risk increased with prolonged duration of PPI use, while in contrast, it declined in H2RA users. In a direct comparison between PPI and H2RA users, PPI users had a higher risk of all-cause mortality compared to H2RA users. The risk of mortality from cancer and cardiovascular disease was increased in PPI users.
The major strength of our study is the population-based design, which included all eligible PPI and H2RA maintenance users, limiting the influence of selection bias and increasing statistical power. Our data were retrieved from validated, high-quality, nationwide registries with overall complete coverage of the entire Swedish population, facilitating the generalisability of obtained results. All PPI use in this study was based on prescriptions ascertained from the Prescribed Drug Registry, minimising the risk of misclassification due to recall bias. Furthermore, we minimised potential confounding by indication by controlling for the indications and using stratified analyses. To account for immortal-time bias, only data based on time since first dispensed prescription were calculated and presented. We used robust methods with a priori written study protocol.
Our study has several limitations. Although we were able to correct for the main confounders (age and sex) and took into account confounding by indication, residual confounding cannot be ruled out. Inherent to the study design, data on conditions that may influence the risk of mortality and lifestyle factors (e.g. smoking, diabetes) were not available for the entire Swedish population, since they are not collected in the nationwide health registries. However, socioeconomic factors and place of residence were unlikely to influence the results, as access to health care should be equal for all Swedish citizens. Additionally, underlying indications for 16.3% of the total users were lacking. It is likely that these participants had more mild and unspecific gastrointestinal symptoms which were less likely recorded while the more severe symptoms were more likely to be recorded [27]. The proportion of PPI users with no indications was relatively smaller than the H2RA users, possibly indicating that PPI users might have expressed more severe symptoms.
We had no direct information on compliance to the prescribed treatment under study, and there was potential for misclassification due to treatment nonadherence. Nevertheless, we only included prescriptions that were dispensed, and the dispensing of repeat prescriptions is strongly suggestive of actual use. We did not have access to data on over the counter (OTC) PPI use. However, PPI packages obtained over the counter are smaller and more expensive [21], making it more likely that PPI maintenance users obtain their PPI doses through prescription rather than over the counter, limiting exposure misclassification. Inherent to the study design, the background population contained exposed participants, which might have diluted the results towards null. Maintenance PPI use is common in Sweden (approximately 11% of all Swedish adults) [25, 27], and thus, finding a comparison group not receiving PPIs or H2RAs, with the same symptoms and indications for treatment is virtually impossible. We had no information on PPI use before July 2005 (start of the Prescribed Drug Registry). Thus, due to this potential left censoring, it is possible that non-PPI users may have received PPIs before the study period, also making it difficult to distinguish between prevalent and incident users. However, we assumed that many of those exposed in 2005 were already maintenance users prior to enrolment in our study.
Our findings of an independent association between PPI use and increased all-cause mortality risk align with previous findings from two cohort studies conducted in Finnish [36] and Italian [37] institutionalised older patients. Their findings are corroborated by the more recent abovementioned VA and UK studies which also found increased all-cause mortality risk among PPI users compared to H2RA users and non-acid suppressant users [3, 22, 23]. Similarly, we found increased all-cause mortality in maintenance H2RA users, relative to the background population. The H2RA users were on average older than the PPI users, and this could explain the increased SMRs observed in H2RA users, considering that the older population had higher frequencies of multiple indications and is more vulnerable to conditions that might result in death [36, 37].
Previous studies have established that the risk of mortality among older patients using PPIs is elevated [3, 17, 22, 23, 36,37,38]. This study confirms this but additionally shows an age-dependent effect of PPI and H2RA use on the relative risk of all-cause and cause-specific mortality which has not been discussed in previous literature [3, 17, 22, 23, 36,37,38]. The higher risk in the younger age groups suggests that the relative impact of PPI use is more prominent in the younger population, an age trend we have also seen consistently in our previous studies looking at the risk of different gastrointestinal cancers in relation to PPI use [8, 25, 27, 39]. The high SMRs may possibly be a result of confounding by indication which seemingly impacts the younger age groups more. The young may seem relatively unlikely to be at risk for serious diseases such as cancer. Symptoms therefore may not be thoroughly investigated at the presence of subclinical disease, which is independently associated with increased risk of death, masking the true association between PPI use and mortality [8, 25, 27, 40]. We also hypothesise that the differences by age could be related to gut microbiome changes, with the gut microbiome of younger individuals potentially being more susceptible to the external environment, particularly maintenance drugs. To our knowledge, this interaction between age and environmental factors on the gut microbiome has not been investigated yet in longitudinal population-based studies; yet the impact of drugs on the microbiome seems far from negligible [41].
PPI use was associated with an increased risk of mortality from cancer and cardiovascular disease, which contributed to the increased overall mortality. A few studies have observed increased risk of cardiovascular-specific mortality associated with PPI use [22, 23], and their findings were corroborated by a recent systematic review [19]. Our findings of increased risk of cancer-specific mortality in PPI users were in line with the results of the VA and Danish cohort studies [20, 22], and not surprising in the light of the accumulating evidence suggesting increased risk of gastrointestinal cancers among PPI users, particularly gastric cancer [18, 42]. The subgroup analyses also showed that the standardised mortality ratios were particularly increased for gastrointestinal cancer, particularly stomach, oesophageal and pancreatic cancer. Our findings may also be explained by increased prescription of PPIs to patients with existing medical problems. A few studies that were able to further examine sub-causes of death, found increased risks for cancer-specific mortality in patients with gastrointestinal cancers [22] such as pancreatic cancers [43], colorectal cancer [44], gastric non-cardia cancer and hepatocellular carcinoma [45].
In line with what was observed in the VA and UK cohort studies [22, 23], PPI use was associated with increased risk of mortality compared with H2RA use in the active comparator analysis. Adjusting for the previously mentioned confounders did not attenuate the effect estimates, thus, suggesting that the increased risk of all-cause mortality with PPI use is less likely explained by confounding by indication. The results were supportive of the independent influence of the PPIs effect per se and seemed to confirm that PPIs are more likely prescribed in more severe cases with comorbid conditions.
Increased risk of mortality was observed for gastrointestinal indications including peptic ulcer disease, gastroduodenitis, H. pylori infection/eradication (in PPI users) and dyspepsia. Interestingly, most available data have demonstrated little to no independent associations between these indications and mortality risk, thus, making it easier to disentangle the effects of these indications from that of their treatment [46,47,48,49]. Additionally, the PPI use-mortality association was also evident in those exposed to aspirin and NSAIDs maintenance treatment. Notably, PPI users without documented indications had an increased risk of all-cause mortality. We analysed this association in an attempt to examine the association of PPI use and risk of death in a presumably lower risk cohort. Our results of increased risk in this population were in line with our a priori expectations and supported the notion that PPIs increase risk of death in the absence of indications (risk factors for mortality). These results together with those from the active comparator analyses further strengthen our resolve that the association cannot only be ascribed to confounding by indication.
The mortality risk in PPI users increased with duration of use in a dose–response relation. At > 5 years duration of use, we observed a 38% increased risk of all-cause mortality, compared with an SMR of 1.29 observed during 1–2.9 years of follow-up. These results were consistent with the Veteran Affairs (VA) studies [3, 22]. In contrast, significantly elevated risks of all-cause mortality in H2RA users were observed only during the first few years of follow-up and decreased with a longer duration of use. These results are more likely due to residual confounding by indication which seems to affect H2RA users more than PPI users.
The Bradford Hill criteria to assess evidence for causality provide a framework that enables us to perform a more comprehensive evaluation of the observed associations. Strength of association was quite considerable in our study especially in the younger age groups, with consistency of data within our analyses and with other studies [3, 17, 22, 23], suggesting internal and external validity. We considered temporality, which we took into account in the study design by excluding all individuals who died within the first year of treatment commencement, ensuring that PPI exposure preceded mortality. The biological gradient was confirmed by the presence of a dose–response relationship for PPI use but not H2RA use, with the risk of mortality increasing with prolonged duration of exposure. Specificity was difficult to prove considering that all-cause mortality is invariably related to many exposures. Various biologically plausible mechanisms for the association of PPI use and mortality have been described by some studies. The VA study [22] highlighted that PPI use is linked to excess cause-specific mortality through worsening of underlying disease or the occurrence of new disease [17, 22]. A US study suggested that long-term exposure to PPIs accelerates endothelial cell ageing resulting in an increase in cardiovascular morbidity and mortality [50]. Mechanisms involving PPIs influence on the microbiome have also been suggested [27, 51,52,53]. Additionally, several mechanisms have been suggested which may explain the association between PPIs and the increased risk of several chronic diseases and cancer, which in turn may also affect mortality [54].
Given the confounding that is inherent to observational studies, our ability to make causal claims is precluded, and there is need to interpret our results with the full cognizance of these limitations. We adjusted for some confounders, yet we acknowledge the presence of residual confounding that we could not control for. Nonetheless, the here shown increased mortality risk among PPI users was consistent across our analyses, even after taking into consideration indication of use, and we also found evidence for a dose–response relationship. The aforementioned limitation should not overshadow the fact that the observed association is of real concern, and our results might have important clinical implications. As abovementioned, our results highlight increased risk especially in the young age groups, therefore, clinicians need to be aware of this greater risk in the young and perform thorough examinations before prescribing PPIs to young people. The same consideration should also be applied in the older age groups where polypharmacy and multiple comorbidities are an issue [17, 22, 24, 36, 37]. Given the high prevalence of maintenance PPI use, clinicians and patients should be aware of possible adverse events associated with maintenance use. Our findings should be used to promote pharmacovigilance and emphasise the importance of exercising cautious use of PPIs where there is a clear medical indication, with limited duration of use.
Conclusion
In conclusion, PPI maintenance use was associated with increased all-cause and cause-specific mortality, and the risk increased with prolonged duration of use. These findings highlight that the growing (possibly inappropriate) use of PPIs might involve potentially hazardous health effects among maintenance users and especially among the younger population.
Abbreviations
- ATC:
-
Anatomical therapeutic chemical
- CI:
-
Confidence interval
- H2RA:
-
Histamine-2 receptor antagonist
- ICD-10:
-
International classification of diseases 10th edition
- IRR/aIRR:
-
Incidence rate ratio/adjusted incidence rate ratio
- NSAIDS:
-
Nonsteroidal anti-inflammatory drugs
- PPI:
-
Proton pump inhibitor
- SMR:
-
Standardised mortality ratio
References
Boghossian TA, Rashid FJ, Thompson W, Welch V, Moayyedi P, Rojas-Fernandez C et al. Deprescribing versus continuation of chronic proton pump inhibitor use in adults. Cochrane Database Syst Rev 2017;3:CD011969.
Kim J, Blackett JW, Jodorkovsky D. Strategies for Effective Discontinuation of Proton Pump Inhibitors. Curr Gastroenterol Rep 2018;20:27.
Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z. Risk of death among users of Proton Pump Inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open 2017;7:e015735.
Savarino V, Marabotto E, Zentilin P, Furnari M, Bodini G, De Maria C et al. Proton pump inhibitors: use and misuse in the clinical setting. Expert Review of Clinical Pharmacology 2018;11:1123–1134.
Daniels B, Pearson SA, Buckley NA, Bruno C, Zoega H. Long-term use of proton-pump inhibitors: whole-of-population patterns in Australia 2013–2016. Therapeutic Advances in Gastroenterology 2020;13:1756284820913743.
Halfdanarson OO, Pottegard A, Bjornsson ES, Lund SH, Ogmundsdottir MH, Steingrimsson E et al. Proton-pump inhibitors among adults: a nationwide drug-utilization study. Therap Adv Gastroenterol 2018;11:1756284818777943.
Lassalle M, Le Tri T, Bardou M, Biour M, Kirchgesner J, Rouby F et al. Use of proton pump inhibitors in adults in France: a nationwide drug utilization study. European Journal of Clinical Pharmacology 2020;76:449–457.
Brusselaers N, Sadr-Azodi O, Engstrand L. Long-term proton pump inhibitor usage and the association with pancreatic cancer in Sweden. Journal of gastroenterology 2020;55:453–461.
Majumdar S. Chronic acid-related disorders are common and underinvestigated. The American Journal of Gastroenterology 2003;98:2409–2414.
Raghunath AS. Review article: the long-term use of proton-pump inhibitors. Alimentary Pharmacology & Therapeutics 2005;22:55–63.
Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. The American journal of gastroenterology. 2013;108:308–328 (quiz 29).
Nehra AK, Alexander JA, Loftus CG, Nehra V. Proton Pump Inhibitors: Review of Emerging Concerns. Mayo Clin Proc 2018;93:240–246.
Forgacs I, Loganayagam A. Overprescribing proton pump inhibitors. BMJ (Clinical Research Ed) 2008;336:2–3.
Lanas A. We Are Using Too Many PPIs, and We Need to Stop: A European Perspective. Am J Gastroenterol 2016;111:1085–1086.
Bjornsson E, Abrahamsson H, Simren M, Mattsson N, Jensen C, Agerforz P et al. Discontinuation of proton pump inhibitors in patients on long-term therapy: a double-blind, placebo-controlled trial. Aliment Pharmacol Ther 2006;24:945–954.
Haastrup PF, Rasmussen S, Hansen JM, Christensen RD, Sondergaard J, Jarbol DE. General practice variation when initiating long-term prescribing of proton pump inhibitors: a nationwide cohort study. BMC Fam Pract 2016;17:57.
Ben-Eltriki M, Green CJ, Maclure M, Musini V, Bassett KL, Wright JM. Do proton pump inhibitors increase mortality? A systematic review and in-depth analysis of the evidence. Pharmacol Res Perspect 2020;8:e00651.
Segna D, Brusselaers N, Glaus D, Krupka N, Misselwitz B. Association between proton-pump inhibitors and the risk of gastric cancer: a systematic review with meta-analysis. Therap Adv Gastroenterol 2021;14:17562848211051464.
Shiraev TP, Bullen A. Proton Pump Inhibitors and Cardiovascular Events: A Systematic Review. Heart Lung Circ 2018;27:443–450.
Tvingsholm SA, Dehlendorff C, Osterlind K, Friis S, Jaattela M. Proton pump inhibitor use and cancer mortality. International Journal of Cancer 2018;143:1315–1326.
Wijarnpreecha K, Thongprayoon C, Chesdachai S, Panjawatanana P, Ungprasert P, Cheungpasitporn W. Associations of Proton-Pump Inhibitors and H2 Receptor Antagonists with Chronic Kidney Disease: A Meta-Analysis. Digestive Diseases and Sciences 2017;62:2821–2827.
Xie Y, Bowe B, Yan Y, Xian H, Li T, Al-Aly Z. Estimates of all cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: cohort study. BMJ (Clinical Research Ed). 2019;365:l1580. www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. Epub 05/31/2019
Brown JP, Tazare JR, Williamson E, Mansfield KE, Evans SJ, Tomlinson LA et al. Proton pump inhibitors and risk of all-cause and cause-specific mortality: A cohort study. British Journal of Clinical Pharmacology 2021;87:3150–3161.
Baik SH, Fung KW, McDonald CJ. The Mortality Risk of Proton Pump Inhibitors in 1.9 Million US Seniors: An Extended Cox Survival Analysis. Clin Gastroenterol Hepatol. 2021. Epub 1/13/2021.
Brusselaers N, Engstrand L, Lagergren J. Maintenance proton pump inhibition therapy and risk of oesophageal cancer. Cancer Epidemiol 2018;53:172–177.
Moayyedi P, Eikelboom JW, Bosch J, Connolly SJ, Dyal L, Shestakovska O et al. Safety of Proton Pump Inhibitors Based on a Large, Multi-Year, Randomized Trial of Patients Receiving Rivaroxaban or Aspirin. Gastroenterology 2019;157:682–91 e2.
Brusselaers N, Wahlin K, Engstrand L, Lagergren J. Maintenance therapy with proton pump inhibitors and risk of gastric cancer: a nationwide population-based cohort study in Sweden. BMJ Open 2017;7:e017739.
Brusselaers N, Lagergren J, Engstrand L. Duration of use of proton pump inhibitors and the risk of gastric and oesophageal cancer. Cancer Epidemiol 2019;62:101585.
Organisation WH. WHO Collaborating Centre for Drug Statistics Methodology, Guidelines for ATC classification and DDD assignment. Oslo 2022.
Brooke HL, Talbäck M, Hörnblad J, Johansson LA, Ludvigsson JF, Druid H et al. The Swedish cause of death register. Eur J Epidemiol 2017;32:765–773.
Statistics Database for Causes of Death [Internet]. 2022 [cited 14/05/2022]. https://sdb.socialstyrelsen.se/if_dor/val.aspx.
Statistical Database Population Statistics [Internet]. 2022 [cited 14/01/2022]. https://www.statistikdatabasen.scb.se/.
Johansson LA, Westerling R. Comparing Swedish hospital discharge records with death certificates: implications for mortality statistics. Int J Epidemiol 2000;29:495–502.
Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450.
Breslow NE, Day NE. Statistical methods in cancer research. Volume I - The analysis of case-control studies. IARC Sci Publ. 1980;5–338
Teramura-Grönblad M, Bell JS, Pöysti MM, Strandberg TE, Laurila JV, Tilvis RS et al. Risk of Death Associated With Use of PPIs in Three Cohorts of Institutionalized Older People in Finland. Journal of the American Medical Directors Association 2012;13:488.e9-e.13.
Maggio M, Corsonello A, Ceda GP, Cattabiani C, Lauretani F, Butto V et al. Proton pump inhibitors and risk of 1-year mortality and rehospitalization in older patients discharged from acute care hospitals. JAMA Intern Med 2013;173:518–523.
Lo CH, Ni P, Yan Y, Ma W, Joshi AD, Nguyen LH, et al. Association of Proton Pump Inhibitor Use With All-Cause and Cause-Specific Mortality. Gastroenterology. 2022 Epub 7/01/2022.
Kamal H, Sadr-Azodi O, Engstrand L, Brusselaers N. Association between Proton Pump Inhibitor Use and Biliary Tract Cancer Risk: a Swedish Population-Based Cohort Study. Hepatology. 2021. Epub 05/22/2021.
Pisanu A, Podda M, Cois A, Uccheddu A. Gastric cancer in the young: is it a different clinical entity? A retrospective cohort study. Gastroenterol Res Pract 2014;2014:125038.
Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, Faust K et al. Population-level analysis of gut microbiome variation. Science. 2016;352:560–564.
Salvo EM, Ferko NC, Cash SB, Gonzalez A, Kahrilas PJ. Umbrella review of 42 systematic reviews with meta-analyses: the safety of proton pump inhibitors. Aliment Pharmacol Ther 2021;54:129–143.
Kearns MD, Boursi B, Yang YX. Proton pump inhibitors on pancreatic cancer risk and survival. Cancer Epidemiology 2017;46:80–84.
Wang X, Liu Q, Halfdanarson OO, Zoega H, Sadr-Azodi O, Engstrand L et al. Proton pump inhibitors and survival in patients with colorectal cancer: a Swedish population-based cohort study. Br J Cancer 2021;125:893–900.
Ayyagari S, Tan MC, Liu Y, El-Serag HB, Thrift AP. Use of Acid-Suppressant Medications After Diagnosis Increases Mortality in a Subset of Gastrointestinal Cancer Patients. Digestive Diseases and Sciences 2020;65:2691–2699.
Chen Y, Segers S, Blaser MJ. Association between Helicobacter pylori and mortality in the NHANES III study. Gut 2013;62:1262–1269.
Islami F, Pourshams A, Nasseri-Moghaddam S, Khademi H, Poutschi H, Khoshnia M et al. Gastroesophageal Reflux Disease and overall and Cause-specific Mortality: A Prospective Study of 50000 Individuals. Middle East J Dig Dis 2014;6:65–80.
Ness-Jensen E, Santoni G, Gottlieb-Vedi E, Lindam A, Pedersen N, Lagergren J. Mortality in gastro-oesophageal reflux disease in a population-based nationwide cohort study of Swedish twins. BMJ Open 2020;10:e037456.
Tsoi KK, Chan FC, Hirai HW, Sung JJ. Risk of gastrointestinal bleeding and benefit from colorectal cancer reduction from long-term use of low-dose aspirin: A retrospective study of 612 509 patients. J Gastroenterol Hepatol 2018;33:1728–1736.
Yepuri G, Sukhovershin R, Nazari-Shafti TZ, Petrascheck M, Ghebre YT, Cooke JP. Proton Pump Inhibitors Accelerate Endothelial Senescence. Circ Res 2016;118:e36-42.
Brusselaers N. Prescribed Drugs and the Microbiome. Gastroenterol Clin North Am. 2019;48:331–342.
Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L et al. Proton pump inhibitors affect the gut microbiome. Gut 2016;65:740–748.
Kamal H, Sadr-Azodi O, Engstrand L, Brusselaers N. Association Between Proton Pump Inhibitor Use and Biliary Tract Cancer Risk: A Swedish Population-Based Cohort Study. Hepatology 2021;74:2021–2031.
Fossmark R, Martinsen TC, Waldum HL. Adverse Effects of Proton Pump Inhibitors-Evidence and Plausibility. Int J Mol Sci 2019;20:5203.
Acknowledgments
We are grateful to the Swedish National Board of Health and Welfare, which collected the data.
Funding
Open access funding provided by Karolinska Institute. This work was supported by the Swedish Research Council (2020-01058).
Author information
Authors and Affiliations
Contributions
Study concept and design: SN, JS and NB; statistical analysis: SN under the supervision of JS and NB; interpretation of data: SN, JS and NB; drafting of manuscript: SN. Critical revision of manuscript and approval for submission: SN, JS and NB. All authors approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ngwenya, S., Simin, J. & Brusselaers, N. Maintenance Proton Pump Inhibitor Use Associated with Increased All-Cause and Cause-Specific Mortality in Sweden. Dig Dis Sci 68, 2252–2263 (2023). https://doi.org/10.1007/s10620-023-07820-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-07820-9