Abstract
Background
Intestinal dysfunction is a common complication of acute pancreatitis. MiR155 may be involved in the occurrence and development of intestinal dysfunction mediated by acute pancreatitis, but the specific mechanism is not clear.
Aims
To investigate the effect of miR155 on severe acute pancreatitis (SAP)-associated intestinal dysfunction and its possible mechanism in a mice model.
Methods
In this study, SAP mice model was induced by intraperitoneal injection of caerulein and LPS in combination. Adeno-associated virus (AAV) was given by tail vein injection before the SAP model. The pancreatic and intestinal histopathology changes were analyzed. Cecal tissue was collected for 16S rRNA Gene Sequencing. Intestinal barrier proteins ZO-1 and E-cad were measured by Immunohistochemistry Staining and Western Blot, respectively. Intestinal tissue miR155 and inflammatory factors TNF-α, IL-1β, and IL-6 were detected by Q-PCR. The expression levels of protein associated with TNF-α and TLR4/MYD88 pathway in the intestinal were detected.
Results
In miR155 overexpression SAP group, the levels of tissue inflammatory factor were significantly increased, intestinal barrier proteins were significantly decreased, and the injury of intestinal was aggravated. Bacterial 16S rRNA sequencing was performed, showing miR155 promotes gut microbiota dysbiosis. The levels of TNF-α, TLR4, and MYD88 in the intestinal were detected, suggesting that miR155 may regulate gut microbiota and activate the TLR4/MYD88 pathway, thereby affecting the release of inflammatory mediators and regulating SAP-related intestinal injury. After application of miR155-sponge, imbalance of intestinal flora and destruction of intestinal barrier-related proteins have been alleviated. The release of inflammatory mediators decreased, and the histopathology injury of intestinal was improved obviously.
Conclusion
MiR155 may play an important role in SAP-associated intestinal dysfunction. MiR155 can significantly alter the intestinal microecology, aggravated intestinal inflammation through TLR4/MYD88 pathway, and disrupts the intestinal barrier in SAP mice.





Similar content being viewed by others
Abbreviations
- SAP:
-
Severe acute pancreatitis
- TLR:
-
Toll-like receptors
- IP:
-
Intraperitoneal
- MYD88:
-
Myeloid differentiation factor 88
- AAV:
-
Adeno-associated virus
- CON:
-
Control group
- CAE-LPS:
-
Caerulein + LPS treatment
- CAE-LPS-miR155-O/E or CAE-LPS-miR155:
-
Caerulein + LPS + miR155 overexpression treatment
- CAE-LPS-miR155-sponge:
-
Caerulein + LPS + miR155 sponge treatment
References
Widdison AL, Karanjia ND. Pancreatic infection complicating acute pancreatitis. Br J Surg 1993;80:148–154.
Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010;139:813–820.
Guo Q, Li A, Xia Q, Hu W. Late infection of pancreatic necrosis: a separate entity in necrotizing pancreatitis with low mortality. Pancreatology 2015;15:360–365.
Tan C, Ling Z, Huang Y, Cao Y, Liu Q, Cai T et al. Dysbiosis of intestinal microbiota associated with inflammation involved in the progression of acute pancreatitis. Pancreas 2015;44:868–875.
Akshintala VS, Talukdar R, Singh VK, Goggins M. The gut microbiome in pancreatic disease. Clin Gastroenterol Hepatol 2019;17:290–295.
Sun Y, He Y, Wang F, Zhang H, de Vos P, Sun J. Low-methoxyl lemon pectin attenuates inflammatory responses and improves intestinal barrier integrity in caerulein-induced experimental acute pancreatitis. Mol Nutr Food Res 2017;61:1600885.
Sonkoly E, Stahle M, Pivarcsi A. MicroRNAs and immunity: novel players in the regulation of normal immune function and inflammation. Semin Cancer Biol 2008;18:131–140.
Mehta A, Baltimore D. MicroRNAs as regulatory elements in immune system logic. Nat Rev Immunol 2016;16:279–294.
Aguilar C, Mano M, Eulalio A. MicroRNAs at the host–bacteria interface: host defense or bacterial offense. Trends Microbiol 2019;27:206–218.
Dalmasso G, Nguyen HT, Yan Y, Laroui H, Charania MA, Ayyadurai S et al. Microbiota modulate host gene expression via microRNAs. PLoS ONE 2011;6:e19293.
Zhu Y, He C, Li X, Cai Y, Hu J, Liao Y et al. Gut microbiota dysbiosis worsens the severity of acute pancreatitis in patients and mice. J Gastroenterol. 2018;54:347–358.
Vigorito E, Kohlhaas S, Lu D, Leyland R. miR-155: an ancient regulator of the immune system. Immunol Rev 2013;253:146–157.
Montagner S, Orlandi EM, Merante S, Monticelli S. The role of miRNAs in mast cells and other innate immune cells. Immunol Rev 2013;253:12–24.
Wan J, Xia L, Xu W, Lu N. Expression and function of miR-155 in diseases of the gastrointestinal tract. Int J Mol Sci 2016;17:709.
Tian R, Wang RL, Xie H, Jin W, Yu KL. Overexpressed miRNA-155 dysregulates intestinal epithelial apical junctional complex in severe acute pancreatitis. World J Gastroenterol 2013;19:8282–8291.
Rothchild AC, Sissons JR, Shafiani S, Plaisier C, Min D, Mai D et al. MiR-155-regulated molecular network orchestrates cell fate in the innate and adaptive immune response to Mycobacterium tuberculosis. Proc Natl Acad Sci USA 2016;113:E6172–E6181.
Xiao B, Liu Z, Li BS, Tang B, Li W, Guo G et al. Induction of microRNA-155 during Helicobacter pylori infection and its negative regulatory role in the inflammatory response. J Infect Dis 2009;200:916–925.
Mifsud EJ, Tan AC, Jackson DC. TLR agonists as modulators of the innate immune response and their potential as agents against infectious disease. Front Immunol 2014;5:79.
Caballero S, Pamer EG. Microbiota-mediated inflammation and antimicrobial defense in the intestine. Annu Rev Immunol 2015;33:227–256.
Wan J, Chen J, Wu D, Yang X, Ouyang Y, Zhu Y et al. Regulation of autophagy affects the prognosis of mice with severe acute pancreatitis. Dig Dis Sci 2018;63:2639–2650. https://doi.org/10.1007/s10620-018-5053-0.
Zhang LJ, Huang XJ, Shi XD, Chen HH, Cui SW, Nie SP. Protective effect of three glucomannans from different plants against DSS induced colitis in female BALB/c mice. Food Funct. 2019;10:1928–1939.
Cen ME, Wang F, Su Y, Zhang WJ, Sun B, Wang G. Gastrointestinal microecology: a crucial and potential target in acute pancreatitis. Apoptosis 2018;23:377–387.
Wu LM, Sankaran SJ, Plank LD, Windsor JA, Petrov MS. Meta-analysis of gut barrier dysfunction in patients with acute pancreatitis. Br J Surg 2014;101:1644–1656.
Wang L, Fouts DE, Starkel P, Hartmann P, Chen P, Llorente C et al. Intestinal REG3 lectins protect against alcoholic steatohepatitis by reducing mucosa-associated microbiota and preventing bacterial translocation. Cell Host Microbe 2016;19:227–239.
Li Q, Wang C, Tang C, Zhao X, He Q, Li J. Identification and characterization of blood and neutrophil-associated microbiomes in patients with severe acute pancreatitis using next-generation sequencing. Front Cell Infect Microbiol 2018;8:5.
Hsin JP, Lu Y, Loeb GB, Leslie CS, Rudensky AY. The effect of cellular context on miR-155-mediated gene regulation in four major immune cell types. Nat Immunol 2018;19:1137–1145.
Chou J, Werb Z. MicroRNAs play a big role in regulating ovarian cancer-associated fibroblasts and the tumor microenvironment. Cancer Discov 2012;2:1078–1080.
Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature 2012;489:220–230.
Liu S, da Cunha AP, Rezende RM, Cialic R, Wei Z, Bry L et al. The host shapes the gut microbiota via fecal microRNA. Cell Host Microbe 2016;19:32–43.
Faraoni I, Antonetti FR, Cardone J, Bonmassar E. miR-155 gene: a typical multifunctional microRNA. Biochim Biophys Acta 2009;1792:497–505.
Wang D, Tang M, Zong P, Liu H, Zhang T, Liu Y et al. MiRNA-155 regulates the Th17/Treg ratio by targeting SOCS1 in severe acute pancreatitis. Front Physiol 2018;9:686.
Liu S, Zou H, Wang Y, Duan X, Chen C, Cheng W et al. miR-155-5p is negatively associated with acute pancreatitis and inversely regulates pancreatic acinar cell progression by targeting rela and Traf3. Cell Physiol Biochem 2018;51:1584–1599.
Tili E, Michaille JJ, Piurowski V, Rigot B, Croce CM. MicroRNAs in intestinal barrier function, inflammatory bowel disease and related cancers-their effects and therapeutic potentials. Curr Opin Pharmacol 2017;37:142–150.
Li Y, Tian Y, Zhu W, Gong J, Guo Z, Guo F et al. IL-10/microRNA-155/SHIP-1 signaling pathway is crucial for commensal bacteria induced spontaneous colitis. Biochem Pharmacol 2016;116:100–106.
Schuster DJ, Dykstra JA, Riedl MS, Kitto KF, Belur LR, McIvor RS et al. Biodistribution of adeno-associated virus serotype 9 (AAV9) vector after intrathecal and intravenous delivery in mouse. Front Neuroanat 2014;8:42.
Zhang M, Zhu HM, He F, Li BY, Li XC. Association between acute pancreatitis and small intestinal bacterial overgrowth assessed by hydrogen breath test. World J Gastroenterol 2017;23:8591–8596.
Mofidi R, Duff MD, Wigmore SJ, Madhavan KK, Garden OJ, Parks RW. Association between early systemic inflammatory response, severity of multiorgan dysfunction and death in acute pancreatitis. Br J Surg 2006;93:738–744.
Kanno H, Nose M, Itoh J, Taniguchi Y, Kyogoku M. Spontaneous development of pancreatitis in the MRL/Mp strain of mice in autoimmune mechanism. Clin Exp Immunol 1992;89:68–73.
Schwaiger T, van den Brandt C, Fitzner B, Zaatreh S, Kraatz F, Dummer A et al. Autoimmune pancreatitis in MRL/Mp mice is a T cell-mediated disease responsive to cyclosporine A and rapamycin treatment. Gut 2014;63:494–505.
Shapira M. Gut microbiotas and host evolution: scaling up symbiosis. Trends Ecol Evol 2016;31:539–549.
Yang S, Li F, Jia S, Zhang K, Jiang W, Shang Y et al. Early secreted antigen ESAT-6 of Mycobacterium Tuberculosis promotes apoptosis of macrophages via targeting the microRNA155-SOCS1 interaction. Cell Physiol Biochem 2015;35:1276–1288.
Etna MP, Sinigaglia A, Grassi A, Giacomini E, Romagnoli A, Pardini M et al. Mycobacterium tuberculosis-induced miR-155 subverts autophagy by targeting ATG3 in human dendritic cells. PLoS Pathog 2018;14:e1006790.
Nahid MA, Satoh M, Chan EK. MicroRNA in TLR signaling and endotoxin tolerance. Cell Mol Immunol 2011;8:388–403.
Bala S, Csak T, Kodys K, Catalano D, Ambade A, Furi I et al. Alcohol-induced miR-155 and HDAC11 inhibit negative regulators of the TLR4 pathway and lead to increased LPS responsiveness of Kupffer cells in alcoholic liver disease. J Leukoc Biol 2017;102:487–498.
Acknowledgments
The National Natural Science Foundation of China (Nos: 81460130 and 81760121), Funding scheme for outstanding young talents in Jiangxi province (20192BCB23021), and the Graduate Innovation Fund of Nanchang University (No. CX2018231) supported this study together.
Author information
Authors and Affiliations
Contributions
JW, XY, and LX contributed to conception and design of the study; XY and JW performed experiment; NSL, CH, YZ, YR, XL, and YZ performed the data analysis; XY and JW drafted the manuscript; NHL and LX performed critical revision of the manuscript; JW, FL, and NHL contributed to reagents/materials/analysis tools; and all authors contributed to manuscript revision and read and approved the submitted version.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10620_2021_7022_MOESM1_ESM.pdf
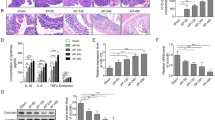
Intestinal barrier destruction and elevated miR155 in mice with SAP (n = 8 for each group). (A) Pancreatic and intestine histology in BALB/c mice. (B) pathology score of intestine. (C) The expression of miR155 and (D) inflammatory index in intestine was shown. Control (CON): saline treatment. CAE+LPS: caerulein +LPS treatment. Data shown are means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 (PDF 585 kb)
Rights and permissions
About this article
Cite this article
Yang, X., Wan, J., Li, N. et al. MiR155 Disrupts the Intestinal Barrier by Inducing Intestinal Inflammation and Altering the Intestinal Microecology in Severe Acute Pancreatitis. Dig Dis Sci 67, 2209–2219 (2022). https://doi.org/10.1007/s10620-021-07022-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-07022-1