Abstract
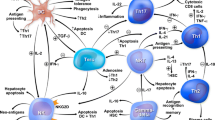
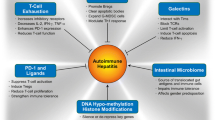
Transforming growth factor-beta and interleukin 10 have diverse immune inhibitory properties that have restored homeostatic defense mechanisms in experimental models of autoimmune disease. The goals of this review are to describe the actions of each cytokine, review their investigational use in animal models and patients, and indicate their prospects as interventions in autoimmune hepatitis. English abstracts were identified in PubMed by multiple search terms. Full-length articles were selected for review, and secondary and tertiary bibliographies were developed. Transforming growth factor-beta expands the natural and inducible populations of regulatory T cells, limits the proliferation of natural killer cells, suppresses the activation of naïve CD8+ T cells, decreases the production of interferon-gamma, and stimulates fibrotic repair. Interleukin 10 selectively inhibits the CD28 co-stimulatory signal for antigen recognition and impairs antigen-specific activation of uncommitted CD4+ and CD8+ T cells. It also inhibits maturation of dendritic cells, suppresses Th17 cells, supports regulatory T cells, and limits production of diverse pro-inflammatory cytokines. Contradictory immune stimulatory effects have been associated with each cytokine and may relate to the dose and accompanying cytokine milieu. Experimental findings have not translated into successful early clinical trials. The recombinant preparation of each agent in low dosage has been safe in human studies. In conclusion, transforming growth factor-beta and interleukin 10 have powerful immune inhibitory actions of potential therapeutic value in autoimmune hepatitis. The keys to their therapeutic application will be to match their predominant non-redundant function with the pivotal pathogenic mechanism or cytokine deficiency and to avoid contradictory immune stimulatory actions.

Similar content being viewed by others
References
Czaja AJ. Transitioning from idiopathic to explainable autoimmune hepatitis. Dig Dis Sci 2015;60:2881–2900. https://doi.org/10.1007/s10620-015-3708-7.
Czaja AJ. Examining pathogenic concepts of autoimmune hepatitis for cues to future investigations and interventions. World J Gastroenterol 2019;25:6579–6606.
Liberal R, Grant CR, Longhi MS, Mieli-Vergani G, Vergani D. Regulatory T cells: mechanisms of suppression and impairment in autoimmune liver disease. IUBMB Life 2015;67:88–97.
Taubert R, Hardtke-Wolenski M, Noyan F et al. Intrahepatic regulatory T cells in autoimmune hepatitis are associated with treatment response and depleted with current therapies. J Hepatol 2014;61:1106–1114.
Czaja AJ, Strettell MD, Thomson LJ et al. Associations between alleles of the major histocompatibility complex and type 1 autoimmune hepatitis. Hepatology 1997;25:317–323.
Czaja AJ. Genetic factors affecting the occurrence, clinical phenotype, and outcome of autoimmune hepatitis. Clin Gastroenterol Hepatol 2008;6:379–388.
van Gerven NM, de Boer YS, Zwiers A et al. HLA-DRB1*03:01 and HLA-DRB1*04:01 modify the presentation and outcome in autoimmune hepatitis type-1. Genes Immun 2015;16:247–252.
Mann DA. Epigenetics in liver disease. Hepatology 2014;60:1418–1425.
Czaja AJ. Epigenetic changes and their implications in autoimmune hepatitis. Eur J Clin Investig 2018;48:e12899.
Oo YH, Hubscher SG, Adams DH. Autoimmune hepatitis: new paradigms in the pathogenesis, diagnosis, and management. Hepatol Int 2010;4:475–493.
Montano-Loza AJ, Czaja AJ. Cell mediators of autoimmune hepatitis and their therapeutic implications. Dig Dis Sci 2014;60:1528–1542. https://doi.org/10.1007/s10620-014-3473-z.
Floreani A, Restrepo-Jimenez P, Secchi MF et al. Etiopathogenesis of autoimmune hepatitis. J Autoimmun 2018;95:133–143.
Trivedi PJ, Adams DH. Mucosal immunity in liver autoimmunity: a comprehensive review. J Autoimmun 2013;46:97–111.
Mack CL, Adams D, Assis DN et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American Association for the Study of Liver Diseases. Hepatology 2020;72:671–722.
O’Garra A, Arai N. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell Biol 2000;10:542–550.
Langrish CL, Chen Y, Blumenschein WM et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 2005;201:233–240.
Chtanova T, Tangye SG, Newton R et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol 2004;173:68–78.
Nurieva RI, Chung Y, Hwang D et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 2008;29:138–149.
Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity 2009;30:324–335.
Seo GY, Youn J, Kim PH. IL-21 ensures TGF-beta 1-induced IgA isotype expression in mouse Peyer’s patches. J Leukoc Biol 2009;85:744–750.
Kelso A. Th1 and Th2 subsets: paradigms lost? Immunol Today 1995;16:374–379.
Ramadori G, Armbrust T. Cytokines in the liver. Eur J Gastroenterol Hepatol 2001;13:777–784.
Yu J, Wei M, Becknell B et al. Pro- and antiinflammatory cytokine signaling: reciprocal antagonism regulates interferon-gamma production by human natural killer cells. Immunity 2006;24:575–590.
Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol 2015;15:271–282.
Del Prete G, De Carli M, Almerigogna F et al. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol 1993;150:353–360.
Akdis CA, Joss A, Akdis M, Faith A, Blaser K. A molecular basis for T cell suppression by IL-10: CD28-associated IL-10 receptor inhibits CD28 tyrosine phosphorylation and phosphatidylinositol 3-kinase binding. FASEB J 2000;14:1666–1668.
Joss A, Akdis M, Faith A, Blaser K, Akdis CA. IL-10 directly acts on T cells by specifically altering the CD28 co-stimulation pathway. Eur J Immunol 2000;30:1683–1690.
Akdis CA, Blaser K. Mechanisms of interleukin-10-mediated immune suppression. Immunology 2001;103:131–136.
Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med 1999;190:995–1004.
Chen W, Jin W, Hardegen N et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 2003;198:1875–1886.
Murai M, Turovskaya O, Kim G et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol 2009;10:1178–1184.
Ouyang W, Beckett O, Ma Q, Li MO. Transforming growth factor-beta signaling curbs thymic negative selection promoting regulatory T cell development. Immunity 2010;32:642–653.
Chaudhry A, Samstein RM, Treuting P et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 2011;34:566–578.
Strainic MG, Shevach EM, An F, Lin F, Medof ME. Absence of signaling into CD4(+) cells via C3aR and C5aR enables autoinductive TGF-beta1 signaling and induction of Foxp3(+) regulatory T cells. Nat Immunol 2013;14:162–171.
Sanjabi S, Oh SA, Li MO. Regulation of the immune response by TGF-beta: from conception to autoimmunity and infection. Cold Spring Harb Perspect Biol 2017;9:a022236.
Gleeson D, Heneghan MA. British Society of Gastroenterology (BSG) guidelines for management of autoimmune hepatitis. Gut 2011;60:1611–1629.
EASL Clinical Practice Guidelines. Autoimmune hepatitis. J Hepatol 2015;63:971–1004.
Czock D, Keller F, Rasche FM, Haussler U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet 2005;44:61–98.
Czaja AJ. Drug choices in autoimmune hepatitis: part A—steroids. Expert Rev Gastroenterol Hepatol 2012;6:603–615.
Fu XQ, Cai JY, Li MJ. Prednisone may rebuild the immunologic homeostasis: alteration of Th17 and Treg cells in the lymphocytes from rats’ spleens after treated with prednisone-containing serum. Mol Genet Genomic Med 2019;7:e00800.
Czaja AJ. Promising pharmacological, molecular and cellular treatments of autoimmune hepatitis. Curr Pharm Des 2011;17:3120–3140.
Liberal R, Krawitt EL, Vierling JM et al. Cutting edge issues in autoimmune hepatitis. J Autoimmun 2016;75:6–19.
Jones D, Manns MP, Terracciano L, Torbenson M, Vierling JM. Unmet needs and new models for future trials in autoimmune hepatitis. Lancet Gastroenterol Hepatol 2018;3:363–370.
Czaja AJ. Review article: opportunities to improve and expand thiopurine therapy for autoimmune hepatitis. Aliment Pharmacol Ther 2020;51:1286–1304.
Vierling JM, Kerkar N, Czaja AJ et al. Immunosuppressive treatment regimens in autoimmune hepatitis: systematic reviews and meta-analyses supporting American Association for the Study of Liver Diseases guidelines. Hepatology 2020;72:753–769.
Czaja AJ. Targeting apoptosis in autoimmune hepatitis. Dig Dis Sci 2014;59:2890–2904. https://doi.org/10.1007/s10620-014-3284-2.
Czaja AJ. Factoring the intestinal microbiome into the pathogenesis of autoimmune hepatitis. World J Gastroenterol 2016;22:9257–9278.
Czaja AJ. Nature and implications of oxidative and nitrosative stresses in autoimmune hepatitis. Dig Dis Sci 2016;61:2784–2803. https://doi.org/10.1007/s10620-016-4247-6.
Czaja AJ. Evolving paradigm for treatment of autoimmune hepatitis. Expert Rev Clin Immunol 2017;13:781–798.
Czaja AJ. Exploring the pathogenic role and therapeutic implications of interleukin 2 in autoimmune hepatitis. Dig Dis Sci. 2020. https://doi.org/10.1007/s10620-020-06562-2.
Halliday N, Dyson JK, Thorburn D, Lohse AW, Heneghan MA. Review article: experimental therapies in autoimmune hepatitis. Aliment Pharmacol Ther 2020;52:1134–1149.
Lucey DR, Clerici M, Shearer GM. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin Microbiol Rev 1996;9:532–562.
Peters M. Actions of cytokines on the immune response and viral interactions: an overview. Hepatology 1996;23:909–916.
Akdis M, Aab A, Altunbulakli C et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: receptors, functions, and roles in diseases. J Allergy Clin Immunol 2016;138:984–1010.
Weiler-Normann C, Schramm C, Quaas A et al. Infliximab as a rescue treatment in difficult-to-treat autoimmune hepatitis. J Hepatol 2013;58:529–534.
Lim TY, Martinez-Llordella M, Kodela E et al. Low-dose interleukin-2 for refractory autoimmune hepatitis. Hepatology 2018;68:1649–1652.
Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol 2006;24:99–146.
Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol 2009;9:447–453.
Oh SA, Li MO. TGF-beta: guardian of T cell function. J Immunol 2013;191:3973–3979.
Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy–review of a new approach. Pharmacol Rev 2003;55:241–269.
Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev 2008;226:205–218.
Wang X, Wong K, Ouyang W, Rutz S. Targeting IL-10 family cytokines for the treatment of human diseases. Cold Spring Harb Perspect Biol 2019;11:a028548.
Neumann C, Scheffold A, Rutz S. Functions and regulation of T cell-derived interleukin-10. Semin Immunol 2019;44:101344.
Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol 2004;4:665–674.
Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol 2004;172:6519–6523.
Arenas-Ramirez N, Woytschak J, Boyman O. Interleukin-2: biology, design and application. Trends Immunol 2015;36:763–777.
Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol 2009;27:485–517.
Zhou L, Lopes JE, Chong MM et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 2008;453:236–240.
Filippi CM, Juedes AE, Oldham JE et al. Transforming growth factor-beta suppresses the activation of CD8+ T-cells when naive but promotes their survival and function once antigen experienced: a two-faced impact on autoimmunity. Diabetes. 2008;7:2684–2692.
Dardalhon V, Awasthi A, Kwon H et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol 2008;9:1347–1355.
Veldhoen M, Uyttenhove C, van Snick J et al. Transforming growth factor-beta “reprograms” the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol 2008;9:1341–1346.
Martinez GJ, Zhang Z, Chung Y et al. Smad3 differentially regulates the induction of regulatory and inflammatory T cell differentiation. J Biol Chem 2009;284:35283–35286.
Gagliani N, Amezcua Vesely MC, Iseppon A et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 2015;523:221–225.
Bettelli E, Carrier Y, Gao W et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006;441:235–238.
Yang L, Anderson DE, Baecher-Allan C et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature 2008;454:350–352.
Lin JT, Martin SL, Xia L, Gorham JD. TGF-beta 1 uses distinct mechanisms to inhibit IFN-gamma expression in CD4+ T cells at priming and at recall: differential involvement of Stat4 and T-bet. J Immunol 2005;174:5950–5958.
Kitani A, Fuss I, Nakamura K et al. Transforming growth factor (TGF)-beta1-producing regulatory T cells induce Smad-mediated interleukin 10 secretion that facilitates coordinated immunoregulatory activity and amelioration of TGF-beta1-mediated fibrosis. J Exp Med 2003;198:1179–1188.
McGeachy MJ, Bak-Jensen KS, Chen Y et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol 2007;8:1390–1397.
Espevik T, Waage A, Faxvaag A, Shalaby MR. Regulation of interleukin-2 and interleukin-6 production from T-cells: involvement of interleukin-1 beta and transforming growth factor-beta. Cell Immunol 1990;126:47–56.
Ahuja SS, Paliogianni F, Yamada H, Balow JE, Boumpas DT. Effect of transforming growth factor-beta on early and late activation events in human T cells. J Immunol 1993;150:3109–3118.
Fargeas C, Wu CY, Nakajima T et al. Differential effect of transforming growth factor beta on the synthesis of Th1- and Th2-like lymphokines by human T lymphocytes. Eur J Immunol 1992;22:2173–2176.
Heath VL, Murphy EE, Crain C, Tomlinson MG, O’Garra A. TGF-beta1 down-regulates Th2 development and results in decreased IL-4-induced STAT6 activation and GATA-3 expression. Eur J Immunol 2000;30:2639–2649.
Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol 1998;16:137–161.
Roberts AB. Molecular and cell biology of TGF-beta. Miner Electrolyte Metab 1998;24:111–119.
Govinden R, Bhoola KD. Genealogy, expression, and cellular function of transforming growth factor-beta. Pharmacol Ther 2003;98:257–265.
Schiller M, Javelaud D, Mauviel A. TGF-beta-induced SMAD signaling and gene regulation: consequences for extracellular matrix remodeling and wound healing. J Dermatol Sci 2004;35:83–92.
Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J 2004;18:816–827.
Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-beta signaling in fibrosis. Growth Factors 2011;29:196–202.
McKaig BC, Hughes K, Tighe PJ, Mahida YR. Differential expression of TGF-beta isoforms by normal and inflammatory bowel disease intestinal myofibroblasts. Am J Physiol Cell Physiol 2002;282:C172-182.
Ask K, Bonniaud P, Maass K et al. Progressive pulmonary fibrosis is mediated by TGF-beta isoform 1 but not TGF-beta3. Int J Biochem Cell Biol 2008;40:484–495.
Cowin AJ, Hatzirodos N, Holding CA et al. Effect of healing on the expression of transforming growth factor beta(s) and their receptors in chronic venous leg ulcers. J Investig Dermatol 2001;117:1282–1289.
Howat WJ, Holgate ST, Lackie PM. TGF-beta isoform release and activation during in vitro bronchial epithelial wound repair. Am J Physiol Lung Cell Mol Physiol 2002;282:L115-123.
Miyazono K, Ten Dijke P, Ichijo H, Heldin CH. Receptors for transforming growth factor-beta. Adv Immunol 1994;55:181–220.
Wang J, Zheng H, Sung CC, Richter KK, Hauer-Jensen M. Cellular sources of transforming growth factor-beta isoforms in early and chronic radiation enteropathy. Am J Pathol 1998;153:1531–1540.
Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol 2010;31:220–227.
Todorovic V, Jurukovski V, Chen Y et al. Latent TGF-beta binding proteins. Int J Biochem Cell Biol 2005;37:38–41.
Marek A, Brodzicki J, Liberek A, Korzon M. TGF-beta (transforming growth factor-beta) in chronic inflammatory conditions—a new diagnostic and prognostic marker? Med Sci Monit 2002;8:RA145–RA151.
Tran DQ, Andersson J, Wang R et al. GARP (LRRC32) is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells. Proc Natl Acad Sci USA 2009;106:13445–13450.
Wang R, Zhu J, Dong X et al. GARP regulates the bioavailability and activation of TGFbeta. Mol Biol Cell 2012;23:1129–1139.
Lyons RM, Keski-Oja J, Moses HL. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol 1988;106:1659–1665.
Dubois CM, Laprise MH, Blanchette F, Gentry LE, Leduc R. Processing of transforming growth factor beta 1 precursor by human furin convertase. J Biol Chem 1995;270:10618–10624.
Pesu M, Watford WT, Wei L et al. T-cell-expressed proprotein convertase furin is essential for maintenance of peripheral immune tolerance. Nature 2008;455:246–250.
Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci 2003;116:217–224.
Jobling MF, Mott JD, Finnegan MT et al. Isoform-specific activation of latent transforming growth factor beta (LTGF-beta) by reactive oxygen species. Radiat Res 2006;166:839–848.
Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev 2000;11:59–69.
Munger JS, Huang X, Kawakatsu H et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999;96:319–328.
Yang Z, Mu Z, Dabovic B et al. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J Cell Biol 2007;176:787–793.
Travis MA, Reizis B, Melton AC et al. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature 2007;449:361–365.
Lacy-Hulbert A, Smith AM, Tissire H et al. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci USA 2007;104:15823–15828.
Melton AC, Bailey-Bucktrout SL, Travis MA et al. Expression of alphavbeta8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice. J Clin Investig 2010;120:4436–4444.
Stockis J, Colau D, Coulie PG, Lucas S. Membrane protein GARP is a receptor for latent TGF-beta on the surface of activated human Treg. Eur J Immunol 2009;39:3315–3322.
Sun L, Jin H, Li H. GARP: a surface molecule of regulatory T cells that is involved in the regulatory function and TGF-beta releasing. Oncotarget 2016;7:42826–42836.
de Jong R, van Lier RA, Ruscetti FW et al. Differential effect of transforming growth factor-beta 1 on the activation of human naive and memory CD4+ T lymphocytes. Int Immunol 1994;6:631–638.
Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev 2005;19:2783–2810.
Takimoto T, Wakabayashi Y, Sekiya T et al. Smad2 and Smad3 are redundantly essential for the TGF-beta-mediated regulation of regulatory T plasticity and Th1 development. J Immunol 2010;185:842–855.
Gu AD, Wang Y, Lin L, Zhang SS, Wan YY. Requirements of transcription factor Smad-dependent and -independent TGF-beta signaling to control discrete T-cell functions. Proc Natl Acad Sci USA 2012;109:905–910.
Kuwahara M, Yamashita M, Shinoda K et al. The transcription factor Sox4 is a downstream target of signaling by the cytokine TGF-beta and suppresses T(H)2 differentiation. Nat Immunol 2012;13:778–786.
Ebner R, Chen RH, Lawler S, Zioncheck T, Derynck R. Determination of type I receptor specificity by the type II receptors for TGF-beta or activin. Science 1993;262:900–902.
Attisano L, Carcamo J, Ventura F et al. Identification of human activin and TGF beta type I receptors that form heteromeric kinase complexes with type II receptors. Cell 1993;75:671–680.
Attisano L, Wrana JL, Lopez-Casillas F, Massague J. TGF-beta receptors and actions. Biochim Biophys Acta 1994;1222:71–80.
Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol 2000;1:169–178.
Rahimi RA, Leof EB. TGF-beta signaling: a tale of two responses. J Cell Biochem 2007;102:593–608.
Wrana JL, Attisano L, Wieser R, Ventura F, Massague J. Mechanism of activation of the TGF-beta receptor. Nature 1994;370:341–347.
Derynck R, Zhang Y, Feng XH. Smads: transcriptional activators of TGF-beta responses. Cell 1998;95:737–740.
Varga J, Pasche B. Transforming growth factor beta as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol 2009;5:200–206.
Chen CH, Seguin-Devaux C, Burke NA et al. Transforming growth factor beta blocks Tec kinase phosphorylation, Ca2+ influx, and NFATc translocation causing inhibition of T cell differentiation. J Exp Med 2003;197:1689–1699.
Szabo SJ, Kim ST, Costa GL et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 2000;100:655–669.
Miller SA, Weinmann AS. Molecular mechanisms by which T-bet regulates T-helper cell commitment. Immunol Rev 2010;238:233–246.
Kallies A, Good-Jacobson KL. Transcription factor T-bet orchestrates lineage development and function in the immune system. Trends Immunol 2017;38:287–297.
Hesslein DG, Lanier LL. Transcriptional control of natural killer cell development and function. Adv Immunol 2011;109:45–85.
Johnson JL, Rosenthal RL, Knox JJ et al. The transcription factor T-bet resolves memory B cell subsets with distinct tissue distributions and antibody specificities in mice and humans. Immunity 2020;52:842–855.
Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med. 2002;195:1499–1505.
Ho IC, Tai TS, Pai SY. GATA3 and the T-cell lineage: essential functions before and after T-helper-2-cell differentiation. Nat Rev Immunol 2009;9:125–135.
Hosoya T, Maillard I, Engel JD. From the cradle to the grave: activities of GATA-3 throughout T-cell development and differentiation. Immunol Rev 2010;238:110–125.
Lentjes MH, Niessen HE, Akiyama Y et al. The emerging role of GATA transcription factors in development and disease. Expert Rev Mol Med 2016;18:e3.
Gorelik L, Fields PE, Flavell RA. Cutting edge: TGF-beta inhibits Th type 2 development through inhibition of GATA-3 expression. J Immunol 2000;165:4773–4777.
Kehrl JH, Roberts AB, Wakefield LM et al. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol 1986;137:3855–3860.
Petit-Koskas E, Genot E, Lawrence D, Kolb JP. Inhibition of the proliferative response of human B lymphocytes to B cell growth factor by transforming growth factor-beta. Eur J Immunol 1988;18:111–116.
Kee BL, Rivera RR, Murre C. Id3 inhibits B lymphocyte progenitor growth and survival in response to TGF-beta. Nat Immunol 2001;2:242–247.
Bouchard C, Fridman WH, Sautes C. Effect of TGF-beta1 on cell cycle regulatory proteins in LPS-stimulated normal mouse B lymphocytes. J Immunol 1997;159:4155–4164.
Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 2006;25:455–471.
Travis MA, Sheppard D. TGF-beta activation and function in immunity. Annu Rev Immunol 2014;32:51–82.
Gorham JD, Lin JT, Sung JL, Rudner LA, French MA. Genetic regulation of autoimmune disease: BALB/c background TGF-beta 1-deficient mice develop necroinflammatory IFN-gamma-dependent hepatitis. J Immunol 2001;166:6413–6422.
Gorham JD. Transforming growth factor-beta1, Th1 responses, and autoimmune liver disease. Transfusion (Paris) 2005;45:51S-59S.
Shull MM, Ormsby I, Kier AB et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 1992;359:693–699.
Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity 2006;25:441–454.
Liu Y, Zhang P, Li J et al. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol 2008;9:632–640.
Zheng Y, Josefowicz S, Chaudhry A et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 2010;463:808–812.
Schlenner SM, Weigmann B, Ruan Q, Chen Y, von Boehmer H. Smad3 binding to the foxp3 enhancer is dispensable for the development of regulatory T cells with the exception of the gut. J Exp Med 2012;209:1529–1535.
Molinero LL, Miller ML, Evaristo C, Alegre ML. High TCR stimuli prevent induced regulatory T cell differentiation in a NF-kappaB-dependent manner. J Immunol 2011;186:4609–4617.
Wei J, Duramad O, Perng OA et al. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci USA 2007;104:18169–18174.
Geissmann F, Revy P, Regnault A et al. TGF-beta 1 prevents the noncognate maturation of human dendritic Langerhans cells. J Immunol 1999;162:4567–4575.
Pallotta MT, Orabona C, Volpi C et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol 2011;12:870–878.
Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol 2013;34:137–143.
Su HC, Leite-Morris KA, Braun L, Biron CA. A role for transforming growth factor-beta 1 in regulating natural killer cell and T lymphocyte proliferative responses during acute infection with lymphocytic choriomeningitis virus. J Immunol 1991;147:2717–2727.
Park YP, Choi SC, Kiesler P et al. Complex regulation of human NKG2D-DAP10 cell surface expression: opposing roles of the gammac cytokines and TGF-beta1. Blood 2011;118:3019–3027.
Sun C, Fu B, Gao Y et al. TGF-beta1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence. PLoS Pathog 2012;8:e1002594.
Castriconi R, Cantoni C, Della Chiesa M et al. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc Natl Acad Sci USA 2003;100:4120–4125.
Mills KH. Induction, function and regulation of IL-17-producing T cells. Eur J Immunol 2008;38:2636–2649.
Esplugues E, Huber S, Gagliani N et al. Control of TH17 cells occurs in the small intestine. Nature 2011;475:514–518.
Chalmin F, Mignot G, Bruchard M et al. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity 2012;36:362–373.
Zhao F, Hoechst B, Gamrekelashvili J et al. Human CCR4+ CCR6+ Th17 cells suppress autologous CD8+ T cell responses. J Immunol 2012;188:6055–6062.
Sharma M, Kaveri SV, Bayry J. Th17 cells, pathogenic or not? TGF-beta3 imposes the embargo. Cell Mol Immunol 2013;10:101–102.
Liu HP, Cao AT, Feng T et al. TGF-beta converts Th1 cells into Th17 cells through stimulation of Runx1 expression. Eur J Immunol 2015;45:1010–1018.
Wei L, Laurence A, Elias KM, O’Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem 2007;282:34605–34610.
Nurieva R, Yang XO, Martinez G et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature 2007;448:480–483.
Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol 2010;40:1830–1835.
Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity 2004;21:467–476.
Fujino S, Andoh A, Bamba S et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 2003;52:65–70.
Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol 2003;171:6173–6177.
Tzartos JS, Friese MA, Craner MJ et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol 2008;172:146–155.
Wong CK, Ho CY, Li EK, Lam CW. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus 2000;9:589–593.
Mangan PR, Harrington LE, O’Quinn DB et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 2006;441:231–234.
Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 2006;24:179–189.
Korn T, Bettelli E, Gao W et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 2007;448:484–487.
Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem 2003;278:1910–1914.
Zhou L, Ivanov II, Spolski R et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol 2007;8:967–974.
Volpe E, Servant N, Zollinger R et al. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol 2008;9:650–657.
McGeachy MJ, Chen Y, Tato CM et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol 2009;10:314–324.
Yang XO, Pappu BP, Nurieva R et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity 2008;28:29–39.
Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol 2008;9:641–649.
Ghoreschi K, Laurence A, Yang XP et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature 2010;467:967–971.
Komatsu N, Mariotti-Ferrandiz ME, Wang Y et al. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci USA 2009;106:1903–1908.
Komatsu N, Okamoto K, Sawa S et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med 2014;20:62–68.
Gao GF, Jakobsen BK. Molecular interactions of coreceptor CD8 and MHC class I: the molecular basis for functional coordination with the T-cell receptor. Immunol Today 2000;21:630–636.
Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol 2002;20:323–370.
Martin MD, Badovinac VP. Defining memory CD8 T cell. Front Immunol 2018;9:2692.
Yu Q, Erman B, Bhandoola A, Sharrow SO, Singer A. In vitro evidence that cytokine receptor signals are required for differentiation of double positive thymocytes into functionally mature CD8+ T cells. J Exp Med 2003;197:475–487.
Brugnera E, Bhandoola A, Cibotti R et al. Coreceptor reversal in the thymus: signaled CD4+8+ thymocytes initially terminate CD8 transcription even when differentiating into CD8+ T cells. Immunity 2000;13:59–71.
Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol 2007;7:144–154.
Ouyang W, Oh SA, Ma Q et al. TGF-beta cytokine signaling promotes CD8+ T cell development and low-affinity CD4+ T cell homeostasis by regulation of interleukin-7 receptor alpha expression. Immunity 2013;39:335–346.
Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 2005;8:369–380.
Pardali K, Moustakas A. Actions of TGF-beta as tumor suppressor and pro-metastatic factor in human cancer. Biochim Biophys Acta 2007;1775:21–62.
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 2002;3:349–363.
Dobaczewski M, Bujak M, Li N et al. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res 2010;107:418–428.
Verrecchia F, Chu ML, Mauviel A. Identification of novel TGF-beta /Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem 2001;276:17058–17062.
Iwano M, Plieth D, Danoff TM et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Investig 2002;110:341–350.
Roberts AB, Tian F, Byfield SD et al. Smad3 is key to TGF-beta-mediated epithelial-to-mesenchymal transition, fibrosis, tumor suppression and metastasis. Cytokine Growth Factor Rev 2006;17:19–27.
Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res 2009;19:156–172.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Investig 2009;119:1420–1428.
Krenning G, Zeisberg EM, Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol 2010;225:631–637.
Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol 2011;179:1074–1080.
Zhao YL, Zhu RT, Sun YL. Epithelial-mesenchymal transition in liver fibrosis. Biomed Rep 2016;4:269–274.
Dooley S, ten Dijke P. TGF-beta in progression of liver disease. Cell Tissue Res 2012;347:245–256.
Czaja MJ, Weiner FR, Flanders KC et al. In vitro and in vivo association of transforming growth factor-beta 1 with hepatic fibrosis. J Cell Biol 1989;108:2477–2482.
Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem 2000;275:2247–2250.
Mauviel A. Transforming growth factor-beta: a key mediator of fibrosis. Methods Mol Med 2005;117:69–80.
Breitkopf K, Godoy P, Ciuclan L, Singer MV, Dooley S. TGF-beta/Smad signaling in the injured liver. Z Gastroenterol 2006;44:57–66.
Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 2008;88:125–172.
Czaja AJ. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J Gastroenterol 2014;20:2515–2532.
Seki E, Brenner DA. Recent advancement of molecular mechanisms of liver fibrosis. J Hepatobiliary Pancreat Sci 2015;22:512–518.
Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology 2015;61:1066–1079.
Nakao A, Afrakhte M, Moren A et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature 1997;389:631–635.
Schnabl B, Kweon YO, Frederick JP et al. The role of Smad3 in mediating mouse hepatic stellate cell activation. Hepatology 2001;34:89–100.
Furukawa F, Matsuzaki K, Mori S et al. p38 MAPK mediates fibrogenic signal through Smad3 phosphorylation in rat myofibroblasts. Hepatology 2003;38:879–889.
Del Castillo G, Murillo MM, Alvarez-Barrientos A et al. Autocrine production of TGF-beta confers resistance to apoptosis after an epithelial-mesenchymal transition process in hepatocytes: role of EGF receptor ligands. Exp Cell Res 2006;312:2860–2871.
Taura K, Miura K, Iwaisako K et al. Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatology 2010;51:1027–1036.
Popov Y, Schuppan D. Epithelial-to-mesenchymal transition in liver fibrosis: dead or alive? Gastroenterology 2010;139:722–725.
Scholten D, Osterreicher CH, Scholten A et al. Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice. Gastroenterology 2010;139:987–998.
Chu AS, Diaz R, Hui JJ et al. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology 2011;53:1685–1695.
Pender SL, Breese EJ, Gunther U et al. Suppression of T cell-mediated injury in human gut by interleukin 10: role of matrix metalloproteinases. Gastroenterology 1998;115:573–583.
Yuan W, Varga J. Transforming growth factor-beta repression of matrix metalloproteinase-1 in dermal fibroblasts involves Smad3. J Biol Chem 2001;276:38502–38510.
Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology 2008;134:1655–1669.
Qureshi HY, Sylvester J, El Mabrouk M, Zafarullah M. TGF-beta-induced expression of tissue inhibitor of metalloproteinases-3 gene in chondrocytes is mediated by extracellular signal-regulated kinase pathway and Sp1 transcription factor. J Cell Physiol 2005;203:345–352.
Qureshi HY, Ricci G, Zafarullah M. Smad signaling pathway is a pivotal component of tissue inhibitor of metalloproteinases-3 regulation by transforming growth factor beta in human chondrocytes. Biochim Biophys Acta 2008;1783:1605–1612.
Radaeva S, Sun R, Jaruga B et al. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology 2006;130:435–452.
Melhem A, Muhanna N, Bishara A et al. Anti-fibrotic activity of NK cells in experimental liver injury through killing of activated HSC. J Hepatol 2006;45:60–71.
Jeong WI, Park O, Suh YG et al. Suppression of innate immunity (natural killer cell/interferon-gamma) in the advanced stages of liver fibrosis in mice. Hepatology 2011;53:1342–1351.
Monteleone G, Kumberova A, Croft NM et al. Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J Clin Investig 2001;108:601–609.
Boirivant M, Pallone F, Di Giacinto C et al. Inhibition of Smad7 with a specific antisense oligonucleotide facilitates TGF-beta1-mediated suppression of colitis. Gastroenterology 2006;131:1786–1798.
Monteleone G, Boirivant M, Pallone F, MacDonald TT. TGF-beta1 and Smad7 in the regulation of IBD. Mucosal Immunol 2008;1:S50-53.
Raz E, Dudler J, Lotz M et al. Modulation of disease activity in murine systemic lupus erythematosus by cytokine gene delivery. Lupus 1995;4:286–292.
Kuruvilla AP, Shah R, Hochwald GM et al. Protective effect of transforming growth factor beta 1 on experimental autoimmune diseases in mice. Proc Natl Acad Sci USA 1991;88:2918–2921.
Thorbecke GJ, Shah R, Leu CH et al. Involvement of endogenous tumor necrosis factor alpha and transforming growth factor beta during induction of collagen type II arthritis in mice. Proc Natl Acad Sci USA 1992;89:7375–7379.
King C, Davies J, Mueller R et al. TGF-beta1 alters APC preference, polarizing islet antigen responses toward a Th2 phenotype. Immunity 1998;8:601–613.
Moritani M, Yoshimoto K, Wong SF et al. Abrogation of autoimmune diabetes in nonobese diabetic mice and protection against effector lymphocytes by transgenic paracrine TGF-beta1. J Clin Investig 1998;102:499–506.
Grewal IS, Grewal KD, Wong FS et al. Expression of transgene encoded TGF-beta in islets prevents autoimmune diabetes in NOD mice by a local mechanism. J Autoimmun 2002;19:9–22.
Racke MK, Dhib-Jalbut S, Cannella B et al. Prevention and treatment of chronic relapsing experimental allergic encephalomyelitis by transforming growth factor-beta 1. J Immunol 1991;146:3012–3017.
Johns LD, Flanders KC, Ranges GE, Sriram S. Successful treatment of experimental allergic encephalomyelitis with transforming growth factor-beta 1. J Immunol 1991;147:1792–1796.
Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001;19:683–765.
Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 2011;29:71–109.
O’Garra A, Vieira P. T(H)1 cells control themselves by producing interleukin-10. Nat Rev Immunol 2007;7:425–428.
Xiao S, Brooks CR, Sobel RA, Kuchroo VK. Tim-1 is essential for induction and maintenance of IL-10 in regulatory B cells and their regulation of tissue inflammation. J Immunol 2015;194:1602–1608.
Czaja AJ. Under-evaluated or unassessed pathogenic pathways in autoimmune hepatitis and implications for future management. Dig Dis Sci 2018;63:1706–1725. https://doi.org/10.1007/s10620-018-5072-x.
DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci 2010;1183:38–57.
Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol 2012;30:221–241.
Bouaziz JD, Le Buanec H, Saussine A, Bensussan A, Bagot M. IL-10 producing regulatory B cells in mice and humans: state of the art. Curr Mol Med 2012;12:519–527.
Yang M, Rui K, Wang S, Lu L. Regulatory B cells in autoimmune diseases. Cell Mol Immunol 2013;10:122–132.
Donnelly RP, Dickensheets H, Finbloom DS. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J Interferon Cytokine Res 1999;19:563–573.
Ho AS, Liu Y, Khan TA et al. A receptor for interleukin 10 is related to interferon receptors. Proc Natl Acad Sci USA 1993;90:11267–11271.
Tan JC, Indelicato SR, Narula SK, Zavodny PJ, Chou CC. Characterization of interleukin-10 receptors on human and mouse cells. J Biol Chem 1993;268:21053–21059.
Pestka S, Krause CD, Sarkar D et al. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol 2004;22:929–979.
Donnelly RP, Sheikh F, Kotenko SV, Dickensheets H. The expanded family of class II cytokines that share the IL-10 receptor-2 (IL-10R2) chain. J Leukoc Biol 2004;76:314–321.
Kotenko SV, Gallagher G, Baurin VV et al. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol 2003;4:69–77.
Sheppard P, Kindsvogel W, Xu W et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol 2003;4:63–68.
Yoon SI, Jones BC, Logsdon NJ et al. Structure and mechanism of receptor sharing by the IL-10R2 common chain. Structure 2010;18:638–648.
Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 2004;39:1332–1342.
Rutz S, Ouyang W. Regulation of interleukin-10 and interleukin-22 expression in T helper cells. Curr Opin Immunol 2011;23:605–612.
Yang X, Zheng SG. Interleukin-22: a likely target for treatment of autoimmune diseases. Autoimmun Rev 2014;13:615–620.
Staples KJ, Smallie T, Williams LM et al. IL-10 induces IL-10 in primary human monocyte-derived macrophages via the transcription factor Stat3. J Immunol 2007;178:4779–4785.
O’Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity 2008;28:477–487.
Greenfield EA, Nguyen KA, Kuchroo VK. CD28/B7 costimulation: a review. Crit Rev Immunol 1998;18:389–418.
Dustin ML, Shaw AS. Costimulation: building an immunological synapse. Science 1999;283:649–650.
Czaja AJ. Understanding the pathogenesis of autoimmune hepatitis. Am J Gastroenterol 2001;96:1224–1231.
Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol 2005;23:515–548.
Cai Z, Brunmark AB, Luxembourg AT et al. Probing the activation requirements for naive CD8+ T cells with Drosophila cell transfectants as antigen presenting cells. Immunol Rev 1998;165:249–265.
Mescher MF, Curtsinger JM, Agarwal P et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev 2006;211:81–92.
Mescher MF, Agarwal P, Casey KA et al. Molecular basis for checkpoints in the CD8 T cell response: tolerance versus activation. Semin Immunol 2007;19:153–161.
Allison JP. CD28-B7 interactions in T-cell activation. Curr Opin Immunol 1994;6:414–419.
Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol 1996;14:233–258.
Curtsinger JM, Schmidt CS, Mondino A et al. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol 1999;162:3256–3262.
Zhang N, Bevan MJ. CD8(+) T cells: foot soldiers of the immune system. Immunity 2011;35:161–168.
Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol 2005;174:4465–4469.
de Waal Malefyt R, Haanen J, Spits H et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med 1991;174:915–924.
de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med 1991;174:1209–1220.
Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature 1992;356:607–609.
Tan P, Anasetti C, Hansen JA et al. Induction of alloantigen-specific hyporesponsiveness in human T lymphocytes by blocking interaction of CD28 with its natural ligand B7/BB1. J Exp Med 1993;177:165–173.
Groux H, Bigler M, de Vries JE, Roncarolo MG. Inhibitory and stimulatory effects of IL-10 on human CD8+ T cells. J Immunol 1998;160:3188–3193.
Rowbottom AW, Lepper MA, Garland RJ, Cox CV, Corley EG. Interleukin-10-induced CD8 cell proliferation. Immunology 1999;98:80–89.
Chikuma S, Bluestone JA. CTLA-4 and tolerance: the biochemical point of view. Immunol Res 2003;28:241–253.
Chikuma S, Abbas AK, Bluestone JA. B7-independent inhibition of T cells by CTLA-4. J Immunol 2005;175:177–181.
Chikuma S. CTLA-4, an essential immune-checkpoint for T-cell activation. Curr Top Microbiol Immunol 2017;410:99–126.
Coomes SM, Kannan Y, Pelly VS et al. CD4(+) Th2 cells are directly regulated by IL-10 during allergic airway inflammation. Mucosal Immunol 2017;10:150–161.
Walker JA, McKenzie ANJ. TH2 cell development and function. Nat Rev Immunol 2018;18:121–133.
Huber S, Gagliani N, Esplugues E et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3(-) and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity 2011;34:554–565.
Wang P, Wu P, Siegel MI, Egan RW, Billah MM. Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem 1995;270:9558–9563.
Schuetze N, Schoeneberger S, Mueller U et al. IL-12 family members: differential kinetics of their TLR4-mediated induction by Salmonella enteritidis and the impact of IL-10 in bone marrow-derived macrophages. Int Immunol 2005;17:649–659.
Maloy KJ, Kullberg MC. IL-23 and Th17 cytokines in intestinal homeostasis. Mucosal Immunol 2008;1:339–349.
Veenbergen S, Li P, Raatgeep HC et al. IL-10 signaling in dendritic cells controls IL-1beta-mediated IFNgamma secretion by human CD4(+) T cells: relevance to inflammatory bowel disease. Mucosal Immunol 2019;12:1201–1211.
Cai G, Kastelein RA, Hunter CA. IL-10 enhances NK cell proliferation, cytotoxicity and production of IFN-gamma when combined with IL-18. Eur J Immunol 1999;29:2658–2665.
Santin AD, Hermonat PL, Ravaggi A et al. Interleukin-10 increases Th1 cytokine production and cytotoxic potential in human papillomavirus-specific CD8(+) cytotoxic T lymphocytes. J Virol 2000;74:4729–4737.
Itoh K, Hirohata S. The role of IL-10 in human B cell activation, proliferation, and differentiation. J Immunol 1995;154:4341–4350.
Levy Y, Brouet JC. Interleukin-10 prevents spontaneous death of germinal center B cells by induction of the bcl-2 protein. J Clin Investig 1994;93:424–428.
Blazar BR, Taylor PA, Smith S, Vallera DA. Interleukin-10 administration decreases survival in murine recipients of major histocompatibility complex disparate donor bone marrow grafts. Blood 1995;85:842–851.
Lauw FN, Pajkrt D, Hack CE et al. Proinflammatory effects of IL-10 during human endotoxemia. J Immunol 2000;165:2783–2789.
Calabresi PA, Fields NS, Maloni HW et al. Phase 1 trial of transforming growth factor beta 2 in chronic progressive MS. Neurology 1998;51:289–292.
Monteleone G, Fantini MC, Onali S et al. Phase I clinical trial of Smad7 knockdown using antisense oligonucleotide in patients with active Crohn’s disease. Mol Ther 2012;20:870–876.
Monteleone G, Neurath MF, Ardizzone S et al. Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn’s disease. N Engl J Med 2015;372:1104–1113.
Marafini I, Monteleone G. Therapeutic oligonucleotides for patients with inflammatory bowel diseases. Biologics 2020;14:47–51.
Feagan BG, Sands BE, Rossiter G et al. Effects of Mongersen (GED-0301) on endoscopic and clinical outcomes in patients with active Crohn’s Disease. Gastroenterology 2018;154:61–64.
Sands BE, Feagan BG, Sandborn WJ et al. Mongersen (GED-0301) for active Crohn’s disease: results of a Phase 3 study. Am J Gastroenterol 2020;115:738–745.
Denton CP, Merkel PA, Furst DE et al. Recombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum 2007;56:323–333.
Sellon RK, Tonkonogy S, Schultz M et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun 1998;66:5224–5231.
Kang SS, Bloom SM, Norian LA et al. An antibiotic-responsive mouse model of fulminant ulcerative colitis. PLoS Med 2008;5:e41.
Quattrocchi E, Dallman MJ, Dhillon AP et al. Murine IL-10 gene transfer inhibits established collagen-induced arthritis and reduces adenovirus-mediated inflammatory responses in mouse liver. J Immunol 2001;166:5970–5978.
Steidler L, Hans W, Schotte L et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 2000;289:1352–1355.
Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993;75:263–274.
Steidler L, Neirynck S, Huyghebaert N et al. Biological containment of genetically modified Lactococcus lactis for intestinal delivery of human interleukin 10. Nat Biotechnol. 2003;21:785–789.
Keystone E, Wherry J, Grint P. IL-10 as a therapeutic strategy in the treatment of rheumatoid arthritis. Rheum Dis Clin N Am 1998;24:629–639.
van Roon JA, Lafeber FP, Bijlsma JW. Synergistic activity of interleukin-4 and interleukin-10 in suppression of inflammation and joint destruction in rheumatoid arthritis. Arthritis Rheum 2001;44:3–12.
van Roon J, Wijngaarden S, Lafeber FP et al. Interleukin 10 treatment of patients with rheumatoid arthritis enhances Fc gamma receptor expression on monocytes and responsiveness to immune complex stimulation. J Rheumatol 2003;30:648–651.
van Deventer SJ, Elson CO, Fedorak RN. Multiple doses of intravenous interleukin 10 in steroid-refractory Crohn’s disease. Crohn’s Disease Study Group. Gastroenterology 1997;113:383–389.
Fedorak RN, Gangl A, Elson CO et al. Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn’s disease. The Interleukin 10 Inflammatory Bowel Disease Cooperative Study Group. Gastroenterology 2000;119:1473–1482.
Schreiber S, Fedorak RN, Nielsen OH et al. Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn’s disease. Crohn’s Disease IL-10 Cooperative Study Group. Gastroenterology 2000;119:1461–1472.
Colombel JF, Rutgeerts P, Malchow H et al. Interleukin 10 (Tenovil) in the prevention of postoperative recurrence of Crohn’s disease. Gut 2001;49:42–46.
Tilg H, van Montfrans C, van den Ende A et al. Treatment of Crohn’s disease with recombinant human interleukin 10 induces the proinflammatory cytokine interferon gamma. Gut 2002;50:191–195.
Asadullah K, Sterry W, Stephanek K et al. IL-10 is a key cytokine in psoriasis. Proof of principle by IL-10 therapy: a new therapeutic approach. J Clin Investig 1998;101:783–794.
Asadullah K, Docke WD, Ebeling M et al. Interleukin 10 treatment of psoriasis: clinical results of a phase 2 trial. Arch Dermatol 1999;135:187–192.
Reich K, Garbe C, Blaschke V et al. Response of psoriasis to interleukin-10 is associated with suppression of cutaneous type 1 inflammation, downregulation of the epidermal interleukin-8/CXCR2 pathway and normalization of keratinocyte maturation. J Investig Dermatol 2001;116:319–329.
McInnes IB, Illei GG, Danning CL et al. IL-10 improves skin disease and modulates endothelial activation and leukocyte effector function in patients with psoriatic arthritis. J Immunol 2001;167:4075–4082.
Kimball AB, Kawamura T, Tejura K et al. Clinical and immunologic assessment of patients with psoriasis in a randomized, double-blind, placebo-controlled trial using recombinant human interleukin 10. Arch Dermatol 2002;138:1341–1346.
Buruiana FE, Sola I, Alonso-Coello P. Recombinant human interleukin 10 for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 2010;11:CD005109.
Demols A, Deviere J. New frontiers in the pharmacological prevention of post-ERCP pancreatitis: the cytokines. JOP 2003;4:49–57.
Lieb JG 2nd, Draganov PV. Early successes and late failures in the prevention of post endoscopic retrograde cholangiopancreatography. World J Gastroenterol 2007;13:3567–3574.
Deviere J, Le Moine O, Van Laethem JL et al. Interleukin 10 reduces the incidence of pancreatitis after therapeutic endoscopic retrograde cholangiopancreatography. Gastroenterology 2001;120:498–505.
Dumot JA, Conwell DL, Zuccaro G Jr et al. A randomized, double blind study of interleukin 10 for the prevention of ERCP-induced pancreatitis. Am J Gastroenterol 2001;96:2098–2102.
Sherman S, Cheng CL, Costamagna G et al. Efficacy of recombinant human interleukin-10 in prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis in subjects with increased risk. Pancreas 2009;38:267–274.
Van Laethem JL, Marchant A, Delvaux A et al. Interleukin 10 prevents necrosis in murine experimental acute pancreatitis. Gastroenterology 1995;108:1917–1922.
Chernoff AE, Granowitz EV, Shapiro L et al. A randomized, controlled trial of IL-10 in humans. Inhibition of inflammatory cytokine production and immune responses. J Immunol 1995;154:5492–5499.
Fuchs AC, Granowitz EV, Shapiro L et al. Clinical, hematologic, and immunologic effects of interleukin-10 in humans. J Clin Immunol 1996;16:291–303.
Bayer EM, Herr W, Kanzler S et al. Transforming growth factor-beta1 in autoimmune hepatitis: correlation of liver tissue expression and serum levels with disease activity. J Hepatol 1998;28:803–811.
Sakaguchi K, Kitano M, Nishimura M et al. Serum level of transforming growth factor-beta1 (TGF-beta1) and the expression of TGF-beta receptor type II in peripheral blood mononuclear cells in patients with autoimmune hepatitis. Hepatogastroenterology 2004;51:1780–1783.
Gutkowski K, Gutkowska D, Kiszka J et al. Serum interleukin17 levels predict inflammatory activity in patients with autoimmune hepatitis. Pol Arch Intern Med 2018;128:150–156.
Schramm C, Protschka M, Kohler HH et al. Impairment of TGF-beta signaling in T cells increases susceptibility to experimental autoimmune hepatitis in mice. Am J Physiol Gastrointest Liver Physiol 2003;284:G525-535.
Paladino N, Flores AC, Fainboim H et al. The most severe forms of type I autoimmune hepatitis are associated with genetically determined levels of TGF-beta1. Clin Immunol 2010;134:305–312.
Dunning AM, Ellis PD, McBride S et al. A transforming growth factorbeta1 signal peptide variant increases secretion in vitro and is associated with increased incidence of invasive breast cancer. Cancer Res 2003;63:2610–2615.
Yokota M, Ichihara S, Lin TL, Nakashima N, Yamada Y. Association of a T29–>C polymorphism of the transforming growth factor-beta1 gene with genetic susceptibility to myocardial infarction in Japanese. Circulation 2000;101:2783–2787.
Bathgate AJ, Pravica V, Perrey C, Hayes PC, Hutchinson IV. Polymorphisms in tumour necrosis factor alpha, interleukin-10 and transforming growth factor beta1 genes and end-stage liver disease. Eur J Gastroenterol Hepatol 2000;12:1329–1333.
Chaouali M, Fernandes V, Ghazouani E, Pereira L, Kochkar R. Association of STAT4, TGFbeta1, SH2B3 and PTPN22 polymorphisms with autoimmune hepatitis. Exp Mol Pathol 2018;105:279–284.
Liberal R, Grant CR, Holder BS et al. In autoimmune hepatitis type 1 or the autoimmune hepatitis-sclerosing cholangitis variant defective regulatory T-cell responsiveness to IL-2 results in low IL-10 production and impaired suppression. Hepatology 2015;62:863–875.
Chen J, Liu W, Zhu W. Foxp3(+) Treg sells are associated with pathological process of autoimmune hepatitis by activating methylation modification in autoimmune hepatitis patients. Med Sci Monit 2019;25:6204–6212.
Czaja AJ, Sievers C, Zein NN. Nature and behavior of serum cytokines in type 1 autoimmune hepatitis. Dig Dis Sci 2000;45:1028–1035. https://doi.org/10.1023/a:1005506031717.
Horan GS, Wood S, Ona V et al. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med 2008;177:56–65.
Puthawala K, Hadjiangelis N, Jacoby SC et al. Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med 2008;177:82–90.
Longhi MS, Ma Y, Mitry RR et al. Effect of CD4+ CD25+ regulatory T-cells on CD8 T-cell function in patients with autoimmune hepatitis. J Autoimmun 2005;25:63–71.
Longhi MS, Ma Y, Bogdanos DP et al. Impairment of CD4(+)CD25(+) regulatory T-cells in autoimmune liver disease. J Hepatol 2004;41:31–37.
Czaja AJ, Carpenter HA. Progressive fibrosis during corticosteroid therapy of autoimmune hepatitis. Hepatology 2004;39:1631–1638.
Czaja AJ. Review article: Prevention and reversal of hepatic fibrosis in autoimmune hepatitis. Aliment Pharmacol Ther 2014;39:385–406.
Mead AL, Wong TT, Cordeiro MF, Anderson IK, Khaw PT. Evaluation of anti-TGF-beta2 antibody as a new postoperative anti-scarring agent in glaucoma surgery. Investig Ophthalmol Vis Sci 2003;44:3394–3401.
Juarez P, Vilchis-Landeros MM, Ponce-Coria J et al. Soluble betaglycan reduces renal damage progression in db/db mice. Am J Physiol Renal Physiol 2007;292:F321-329.
Petersen M, Thorikay M, Deckers M et al. Oral administration of GW788388, an inhibitor of TGF-beta type I and II receptor kinases, decreases renal fibrosis. Kidney Int 2008;73:705–715.
Itoh S, ten Dijke P. Negative regulation of TGF-beta receptor/Smad signal transduction. Curr Opin Cell Biol 2007;19:176–184.
Lan HY. Smad7 as a therapeutic agent for chronic kidney diseases. Front Biosci 2008;13:4984–4992.
Montano-Loza AJ, Thandassery RB, Czaja AJ. Targeting hepatic fibrosis in autoimmune hepatitis. Dig Dis Sci 2016;61:3118–3139. https://doi.org/10.1007/s10620-016-4254-7.
Czaja AJ, Manns MP. Advances in the diagnosis, pathogenesis and management of autoimmune hepatitis. Gastroenterology 2010;139:58–72.
Burak KW, Swain MG, Santodomino-Garzon T et al. Rituximab for the treatment of patients with autoimmune hepatitis who are refractory or intolerant to standard therapy. Can J Gastroenterol 2013;27:273–280.
D’Agostino D, Costaguta A, Alvarez F. Successful treatment of refractory autoimmune hepatitis with rituximab. Pediatrics 2013;132:e526-530.
Smith KA. Interleukin-2: inception, impact, and implications. Science 1988;240:1169–1176.
Nelson BH. IL-2, regulatory T cells, and tolerance. J Immunol 2004;172:3983–3988.
Willems F, Marchant A, Delville JP et al. Interleukin-10 inhibits B7 and intercellular adhesion molecule-1 expression on human monocytes. Eur J Immunol 1994;24:1007–1009.
Creery WD, Diaz-Mitoma F, Filion L, Kumar A. Differential modulation of B7–1 and B7–2 isoform expression on human monocytes by cytokines which influence the development of T helper cell phenotype. Eur J Immunol 1996;26:1273–1277.
Funding
This review did not receive financial support from a funding agency or institution.
Author information
Authors and Affiliations
Contributions
AJC, MD, researched, designed, and wrote this article. The tables and color figure are original, constructed by Dr. Czaja, and developed solely for this review. The review article is original, current, and comprehensive, and it has not been published previously.
Corresponding author
Ethics declarations
Conflict of interest
Albert J. Czaja, MD, has no conflict of interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Czaja, A.J. Immune Inhibitory Properties and Therapeutic Prospects of Transforming Growth Factor-Beta and Interleukin 10 in Autoimmune Hepatitis. Dig Dis Sci 67, 1163–1186 (2022). https://doi.org/10.1007/s10620-021-06968-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-06968-6