Abstract
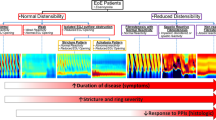
Recent innovations in esophageal diagnostic testing have enhanced gastroenterology clinical practice by facilitating more nuanced and advanced evaluation of esophageal symptoms. Among these pivotal advances is the FDA-approved functional lumen imaging probe (FLIP), which utilizes impedance planimetry via volumetric distension of a catheter-mounted balloon at the time of sedated upper endoscopy, to acquire esophageal dimensions and pressures. In real time, FLIP can display cross-sectional areas (CSA) and distensibility indices (ratios of CSA to intra-balloon pressures) throughout the esophagus, most notably at the esophagogastric junction, as well as secondary peristaltic esophageal body contractile patterns. As the use of FLIP has progressively spread and permeated into the practice of clinical gastroenterology since its introduction, increasing data on and experiences with its applications have accumulated to guide its utility in clinical practice. In this current review developed for gastroenterologists and foregut surgeons across clinical practice, we provide an introduction to FLIP technology and metrics and discuss the clinical scenarios in which performance of or referral for FLIP may be helpful in the evaluation and management of patients with commonly encountered esophageal symptoms and disorders. Specifically, we discuss the potential applications and limitations of FLIP as a complementary diagnostic modality in patients with non-obstructive dysphagia, established or suspected achalasia spectrum disorders, eosinophilic esophagitis, gastroesophageal reflux disease and those undergoing esophageal surgery.


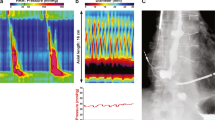
The authors thank Dr. C. Prakash Gyawali from the Washington University School of Medicine for providing the image for c
Similar content being viewed by others
Abbreviations
- CSA:
-
Cross-sectional area
- DI:
-
Distensibility index
- EGJ:
-
Esophagogastric junction
- EGJOO:
-
Esophagogastric junction outflow obstruction
- EOE:
-
Eosinophilic esophagitis
- FLIP:
-
Functional lumen imaging probe
- GERD:
-
Gastroesophageal reflux disease
- HRM:
-
High-resolution manometry
- IRP:
-
Integrated relaxation pressure
- LES:
-
Lower esophageal sphincter
- POEM:
-
Per-oral endoscopic myotomy
- RAC:
-
Repetitive antegrade contractions
- RRC:
-
Repetitive retrograde contractions
References
Gyawali CP, Carlson D, Chen J, Patel A, Wong R, Yadlapati R. American College of Gastroenterology clinical guideline: clinical use of esophageal physiologic testing. Am J Gastroenterol. 2020. (in press).
Gyawali CP. High resolution manometry: the Ray Clouse legacy. Neurogastroenterol Motil. 2012;24:2–4.
Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160–174.
Dhawan I, O’Connell B, Patel A, et al. Utility of esophageal high-resolution manometry in clinical practice: first, do HRM. Dig Dis Sci. 2018;63:3178–3186. https://doi.org/10.1007/s10620-018-5300-4.
Patel A, Sayuk GS, Gyawali CP. Parameters on esophageal pH-impedance monitoring that predict outcomes of patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2015;13:884–891.
Patel A, Sayuk GS, Kushnir VM, et al. GERD phenotypes from pH-impedance monitoring predict symptomatic outcomes on prospective evaluation. Neurogastroenterol Motil. 2016;28:513–521.
Gyawali CP, Kahrilas PJ, Savarino E, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut. 2018;67:1351–1362.
Ayazi S, Lipham JC, Portale G, et al. Bravo catheter-free pH monitoring: normal values, concordance, optimal diagnostic thresholds, and accuracy. Clin Gastroenterol Hepatol. 2009;7:60–67.
Frazzoni M, Savarino E, de Bortoli N, et al. Analyses of the post-reflux swallow-induced peristaltic wave index and nocturnal baseline impedance parameters increase the diagnostic yield of impedance-pH monitoring of patients with reflux disease. Clin Gastroenterol Hepatol. 2016;14:40–46.
Patel A, Wang D, Sainani N, et al. Distal mean nocturnal baseline impedance on pH-impedance monitoring predicts reflux burden and symptomatic outcome in gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2016;44:890–898.
Patel D, Higginbotham T, Slaughter J, et al. Development and validation of a mucosal impedance contour analysis system to distinguish esophageal disorders. Gastroenterology. 2019;156:1617.
Rengarajan A, Savarino E, Della Coletta M, et al. Mean nocturnal baseline impedance correlates with symptom outcome when acid exposure time is inconclusive on esophageal reflux monitoring. Clin Gastroenterol Hepatol. 2020;18:589–595.
de Bortoli N, Martinucci I, Savarino E, et al. Association between baseline impedance values and response proton pump inhibitors in patients with heartburn. Clin Gastroenterol Hepatol. 2015;13:1082-8e1.
Hirano I, Pandolfino J, Boeckxstaens G. Functional lumen imaging probe for the management of esophageal disorders: expert review from the clinical practice updates committee of the AGA Institute. Clin Gastroenterol Hepatol. 2017;15:325–334.
Gregersen H, Stodkilde-Jorgensen H, Djurhuus JC, et al. The four-electrode impedance technique: a method for investigation of compliance in luminal organs. Clin Phys Physiol Meas. 1988;9:61–64.
Gregersen H, Djurhuus JC. Impedance planimetry: a new approach to biomechanical intestinal wall properties. Dig Dis. 1991;9:332–340.
Orvar KB, Gregersen H, Christensen J. Biomechanical characteristics of the human esophagus. Dig Dis Sci. 1993;38:197–205. https://doi.org/10.1007/BF01307535.
Rao SS, Hayek B, Summers RW. Impedance planimetry: an integrated approach for assessing sensory, active, and passive biomechanical properties of the human esophagus. Am J Gastroenterol. 1995;90:431–438.
Rao SS, Gregersen H, Hayek B, et al. Unexplained chest pain: the hypersensitive, hyperreactive, and poorly compliant esophagus. Ann Intern Med. 1996;124:950–958.
Pandolfino JE, Shi G, Trueworthy B, et al. Esophagogastric junction opening during relaxation distinguishes nonhernia reflux patients, hernia patients, and normal subjects. Gastroenterology. 2003;125:1018–1024.
McMahon B, Frøkjær JB, Drewes AM, et al. A new measurement of oesophago-gastric junction competence. Neurogastroenterol Motil. 2004;16:543–546.
Kwiatek MA, Kahrilas PJ, Soper NJ, et al. Esophagogastric junction distensibility after fundoplication assessed with a novel functional luminal imaging probe. J Gastrointest Surg. 2010;14:268–276.
Rohof WO, Hirsch DP, Kessing BF, et al. Efficacy of treatment for patients with achalasia depends on the distensibility of the esophagogastric junction. Gastroenterology. 2012;143:328–335.
Pandolfino JE, de Ruigh A, Nicodème F, et al. Distensibility of the esophagogastric junction assessed with the functional lumen imaging probe (FLIP™) in achalasia patients. Neurogastroenterol Motil. 2013;25:496-e368.
Kwiatek MA, Pandolfino JE, Hirano I, et al. Esophagogastric junction distensibility assessed with an endoscopic functional luminal imaging probe (EndoFLIP). Gastrointest Endosc. 2010;72:272–278.
Bianca A, Schindler V, Schnurre L, et al. Endoscope presence during endoluminal functional lumen imaging probe (FLIP) influences FLIP metrics in the evaluation of esophageal dysmotility. Neurogastroenterol Motil. 2020;32:e13823.
Pandolfino JE, de Ruigh A, Nicodeme F, et al. Distensibility of the esophagogastric junction assessed with the functional lumen imaging probe (FLIP) in achalasia patients. Neurogastroenterol Motil. 2013;25:496–501.
Carlson DA, Lin Z, Kahrilas PJ, et al. The functional lumen imaging probe detects esophageal contractility not observed with manometry in patients with achalasia. Gastroenterology. 2015;149:1742–1751.
Carlson DA, Kahrilas PJ, Lin Z, et al. Evaluation of esophageal motility utilizing the functional lumen imaging probe. Am J Gastroenterol. 2016;111:1726–1735.
Carlson DA, Kou W, Lin Z, et al. Normal values of esophageal distensibility and distension-induced contractility measured by functional luminal imaging probe panometry. Clin Gastroenterol Hepatol. 2019;17:674–681.e1.
Triggs JR, Carlson DA, Beveridge C, et al. Functional luminal imaging probe panometry identifies achalasia-type esophagogastric junction outflow obstruction. Clin Gastroenterol Hepatol. 2019. https://doi.org/10.1016/j.cgh.2019.11.037.
Schatzki R. The lower esophageal ring. Long term follow-up of symptomatic and asymptomatic rings. Am J Roentgenol Radium Ther Nucl Med. 1963;90:805–810.
Carlson DA, Hirano I, Zalewski A, et al. Improvement in esophageal distensibility in response to medical and diet therapy in eosinophilic esophagitis. Clin Transl Gastroenterol. 2017;8:e119.
Rooney KP, Baumann AJ, Donnan E, et al. Esophagogastric junction opening parameters are consistently abnormal in untreated achalasia. Clin Gastroenterol Hepatol. 2020. https://doi.org/10.1016/j.cgh.2020.03.069.
Carlson DA, Gyawali CP, Kahrilas PJ, et al. Esophageal motility classification can be established at the time of endoscopy: a study evaluating real-time functional luminal imaging probe panometry. Gastrointest Endosc. 2019;90:915–923.e1.
Baumann AJ, Donnan EN, Triggs JR, et al. Normal functional luminal imaging probe panometry findings associate with lack of major esophageal motility disorder on high-resolution manometry. Clin Gastroenterol Hepatol. 2020. https://doi.org/10.1016/j.cgh.2020.03.040.
Patel A, Posner S, Gyawali CP. Esophageal high-resolution manometry in gastroesophageal reflux disease. Jama. 2018;320:1279–1280.
Khashab MA, Vela MF, Thosani N, et al. ASGE guideline on the management of achalasia. Gastrointest Endosc. 2020;91:213–227.e6.
Oude Nijhuis RAB, Zaninotto G, Roman S, et al. European guidelines on achalasia: United European Gastroenterology and European Society of Neurogastroenterology and Motility recommendations. United Eur Gastroenterol J. 2020;8:13–33.
Rieder E, Swanstrom LL, Perretta S, et al. Intraoperative assessment of esophagogastric junction distensibility during per oral endoscopic myotomy (POEM) for esophageal motility disorders. Surg Endosc. 2013;27:400–405.
Chen JW, Rubenstein JH. Esophagogastric junction distensibility assessed using the functional lumen imaging probe. World J Gastroenterol. 2017;23:1289–1297.
Ponds FA, Bredenoord AJ, Kessing BF, et al. Esophagogastric junction distensibility identifies achalasia subgroup with manometrically normal esophagogastric junction relaxation. Neurogastroenterol Motil. 2017;29:e12908.
Kim E, Yoo IK, Yon DK, et al. Characteristics of a subset of achalasia with normal integrated relaxation pressure. J Neurogastroenterol Motil. 2020;26:274–280.
Carlson DA, Kou W, Pandolfino JE. The rhythm and rate of distension-induced esophageal contractility: a physiomarker of esophageal function. Neurogastroenterol Motil. 2020;32:e13794.
Campagna RAJ, Carlson DA, Hungness ES, et al. Intraoperative assessment of esophageal motility using FLIP during myotomy for achalasia. Surg Endosc. 2019;34:2593–2600.
Teitelbaum EN, Soper NJ, Pandolfino JE, et al. Esophagogastric junction distensibility measurements during Heller myotomy and POEM for achalasia predict postoperative symptomatic outcomes. Surg Endosc. 2015;29:522–528.
Jain AS, Carlson DA, Triggs J, et al. Esophagogastric junction distensibility on functional lumen imaging probe topography predicts treatment response in achalasia-anatomy matters! Am J Gastroenterol. 2019;114:1455–1463.
Verlaan T, Rohof WO, Bredenoord AJ, et al. Effect of peroral endoscopic myotomy on esophagogastric junction physiology in patients with achalasia. Gastrointest Endosc. 2013;78:39–44.
Yoo IK, Choi SA, Kim WH, et al. Assessment of clinical outcomes after peroral endoscopic myotomy via esophageal distensibility measurements with the endoluminal functional lumen imaging probe. Gut Liver. 2019;13:32–39.
Perretta S, Dallemagne B, Allemann P, et al. Multimedia manuscript. Heller myotomy and intraluminal fundoplication: a NOTES technique. Surg Endosc. 2010;24:2903.
Perretta S, Dallemagne B, Donatelli G, et al. Transoral endoscopic esophageal myotomy based on esophageal function testing in a survival porcine model. Gastrointest Endosc. 2011;73:111–116.
Su B, Callahan ZM, Novak S, et al. Using impedance planimetry (EndoFLIP) to evaluate myotomy and predict outcomes after surgery for achalasia. J Gastrointest Surg. 2020;24:964–971.
Teitelbaum EN, Boris L, Arafat FO, et al. Comparison of esophagogastric junction distensibility changes during POEM and Heller myotomy using intraoperative FLIP. Surg Endosc. 2013;27:4547–4555.
Wu PI, Szczesniak MM, Craig PI, et al. Novel intra-procedural distensibility measurement accurately predicts immediate outcome of pneumatic dilatation for idiopathic achalasia. Am J Gastroenterol. 2018;113:205–212.
Kappelle WF, Bogte A, Siersema PD. Hydraulic dilation with a shape-measuring balloon in idiopathic achalasia: a feasibility study. Endoscopy. 2015;47:1028–1034.
Hirano I, Chan ES, Rank MA, et al. AGA institute and the joint task force on allergy-immunology practice parameters clinical guidelines for the management of eosinophilic esophagitis. Gastroenterology. 2020;158:1776–1786.
Posner S, Boyd A, Patel A. Dysphagia in a 34-year-old woman. JAMA. 2020;323:660–661. https://doi.org/10.1001/jama.2019.19121.
Gentile N, Katzka D, Ravi K, et al. Oesophageal narrowing is common and frequently under-appreciated at endoscopy in patients with oesophageal eosinophilia. Aliment Pharmacol Ther. 2014;40:1333–1340.
Kwiatek MA, Hirano I, Kahrilas PJ, et al. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology. 2011;140:82–90.
Lin Z, Kahrilas PJ, Xiao Y, et al. Functional luminal imaging probe topography: an improved method for characterizing esophageal distensibility in eosinophilic esophagitis. Therap Adv Gastroenterol. 2013;6:97–107.
Carlson DA, Lin Z, Hirano I, et al. Evaluation of esophageal distensibility in eosinophilic esophagitis: an update and comparison of functional lumen imaging probe analytic methods. Neurogastroenterol Motil. 2016;28:1844–1853.
Nicodeme F, Hirano I, Chen J, et al. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2013;11:1101–1107.e1.
Menard-Katcher C, Benitez AJ, Pan Z, et al. Influence of age and eosinophilic esophagitis on esophageal distensibility in a pediatric cohort. Am J Gastroenterol. 2017;112:1466–1473.
Chen JW, Pandolfino JE, Lin Z, et al. Severity of endoscopically identified esophageal rings correlates with reduced esophageal distensibility in eosinophilic esophagitis. Endoscopy. 2016;48:794–801.
Ng K, Mogul D, Hollier J, et al. Utility of functional lumen imaging probe in esophageal measurements and dilations: a single pediatric center experience. Surg Endosc. 2020;34:1294–1299.
Wu PI, Szczesniak MM, Maclean J, et al. Clinical utility of a functional lumen imaging probe in management of dysphagia following head and neck cancer therapies. Endoscopy. 2017;49:848–854.
Gyawali CP, Roman S, Bredenoord AJ, et al. Classification of esophageal motor findings in gastro-esophageal reflux disease: conclusions from an international consensus group. Neurogastroenterol Motil. 2017;29:e13104.
Lottrup C, McMahon BP, Ejstrud P, et al. Esophagogastric junction distensibility in hiatus hernia. Dis Esophagus. 2016;29:463–471.
Tucker E, Sweis R, Anggiansah A, et al. Measurement of esophago-gastric junction cross-sectional area and distensibility by an endolumenal functional lumen imaging probe for the diagnosis of gastro-esophageal reflux disease. Neurogastroenterol Motil. 2013;25:904–910.
Carlson DA, Kathpalia P, Craft J, et al. The relationship between esophageal acid exposure and the esophageal response to volumetric distention. Neurogastroenterol Motil. 2018;30:e13240.
DeHaan RK, Davila D, Frelich MJ, et al. Esophagogastric junction distensibility is greater following Toupet compared to Nissen fundoplication. Surg Endosc. 2017;31:193–198.
Hoppo T, McMahon BP, Witteman BP, et al. Functional lumen imaging probe to assess geometric changes in the esophagogastric junction following endolumenal fundoplication. J Gastrointest Surg. 2011;15:1112–1120.
Ilczyszyn A, Botha AJ. Feasibility of esophagogastric junction distensibility measurement during Nissen fundoplication. Dis Esophagus. 2014;27:637–644.
Su B, Novak S, Callahan ZM, et al. Using impedance planimetry (EndoFLIP) in the operating room to assess gastroesophageal junction distensibility and predict patient outcomes following fundoplication. Surg Endosc. 2020;34:1761–1768.
Kim MP, Meisenbach LM, Chan EY. Tailored fundoplication with endoluminal functional lumen imaging probe allows for successful minimally invasive hiatal hernia repair. Surg Laparosc Endosc Percutan Tech. 2018;28:178–182.
Carlson DA, Kahrilas PJ, Ritter K, et al. Mechanisms of repetitive retrograde contractions in response to sustained esophageal distension: a study evaluating patients with postfundoplication dysphagia. Am J Physiol Gastrointest Liver Physiol. 2018;314:G334–G340.
Ahuja NK, Agnihotri A, Lynch KL, et al. Esophageal distensibility measurement: impact on clinical management and procedure length. Dis Esophagus. 2017;30:1–8.
Regan J, Walshe M, Rommel N, et al. A new evaluation of the upper esophageal sphincter using the functional lumen imaging probe: a preliminary report. Dis Esophagus. 2013;26:117–123.
Regan J, Walshe M, Rommel N, et al. New measures of upper esophageal sphincter distensibility and opening patterns during swallowing in healthy subjects using EndoFLIP(R). Neurogastroenterol Motil. 2013;25:e25–e34.
Regan J, Walshe M, Timon C, et al. EndoFLIP(R) evaluation of pharyngo-oesophageal segment tone and swallowing in a clinical population: a total laryngectomy case series. Clin Otolaryngol. 2015;40:121–129.
Malik Z, Sankineni A, Parkman HP. Assessing pyloric sphincter pathophysiology using EndoFLIP in patients with gastroparesis. Neurogastroenterol Motil. 2015;27:524–531.
Snape WJ, Lin MS, Agarwal N, et al. Evaluation of the pylorus with concurrent intraluminal pressure and EndoFLIP in patients with nausea and vomiting. Neurogastroenterol Motil. 2016;28:758–764.
Gourcerol G, Tissier F, Melchior C, et al. Impaired fasting pyloric compliance in gastroparesis and the therapeutic response to pyloric dilatation. Aliment Pharmacol Ther. 2015;41:360–367.
Desprez C, Melchior C, Wuestenberghs F, et al. Pyloric distensibility measurement predicts symptomatic response to intrapyloric botulinum toxin injection. Gastrointest Endosc. 2019;90:754–760.e1.
Yu JX, Baker JR, Watts L, et al. Functional lumen imaging probe is useful for the quantification of gastric sleeve stenosis and prediction of response to endoscopic dilation: a pilot study. Obes Surg. 2020;30:786–789.
Andersen IS, Gregersen H, Buntzen S, et al. New probe for the measurement of dynamic changes in the rectum. Neurogastroenterol Motil. 2004;16:99–105.
Luft F, Fynne L, Gregersen H, et al. Functional luminal imaging probe: a new technique for dynamic evaluation of mechanical properties of the anal canal. Tech Coloproctol. 2012;16:451–457.
Sorensen G, Liao D, Lundby L, et al. Distensibility of the anal canal in patients with idiopathic fecal incontinence: a study with the functional lumen imaging probe. Neurogastroenterol Motil. 2014;26:255–263.
Haas S, Liao D, Gregersen H, et al. Increased yield pressure in the anal canal during sacral nerve stimulation: a pilot study with the functional lumen imaging probe. Neurogastroenterol Motil. 2017;29:e12929.
Zifan A, Sun C, Gourcerol G, et al. EndoFLIP versus high-definition manometry in the assessment of fecal incontinence: a data-driven unsupervised comparison. Neurogastroenterol Motil. 2018;30:e13462.
Gourcerol G, Granier S, Bridoux V, et al. Do EndoFLIP assessments of anal sphincter distensibility provide more information on patients with fecal incontinence than high-resolution anal manometry? Neurogastroenterol Motil. 2016;28:399–409.
Leroi AM, Melchior C, Charpentier C, et al. The diagnostic value of the functional lumen imaging probe versus high-resolution anorectal manometry in patients with fecal incontinence. Neurogastroenterol Motil. 2018;30:e13291.
Zifan A, Mittal RK, Kunkel DC, et al. Loop analysis of the anal sphincter complex in fecal incontinent patients using functional luminal imaging probe. Am J Physiol Gastrointest Liver Physiol. 2020;318:G66–G76.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dorsey, Y.C., Posner, S. & Patel, A. Esophageal Functional Lumen Imaging Probe (FLIP): How Can FLIP Enhance Your Clinical Practice?. Dig Dis Sci 65, 2473–2482 (2020). https://doi.org/10.1007/s10620-020-06443-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06443-8