Abstract
Background
Few studies have examined the metabolic consequences of short bowel syndrome (SBS) and its effects on body composition in adults. We hypothesized that body composition of SBS patients is altered compared to a normal age-, race-, and sex-matched population, regardless of parenteral nutrition (PN) dependence.
Aim
To compare the body composition of adult patients with SBS to age-, sex-, and race-matched healthy controls.
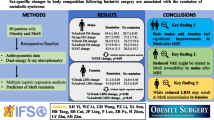
Methods
Twenty patients with SBS underwent body composition analysis using the GE Lunar iDXA scanner. Patients were age-, sex-, and race-matched to controls from the National Health and Nutrition Examination Survey (1999–2004). Mean differences in body mass index, fat-free mass, fat mass, percent body fat, visceral adipose tissue mass and volume, and bone mineral density were measured. Statistical analysis was performed using SAS 9.4 software.
Results
Fifty-five percent of subjects had a history of PN use, and 30% were current PN users. Mean percent body fat for SBS patients was 35.1% compared to 30.9% for healthy controls (p = 0.043). Fat-free mass was reduced in SBS (p = 0.007). Patients with reduced bone mass had a trend toward significantly more years of PN exposure compared to those with normal bone mass (p = 0.094), and a trend toward older age (p = 0.075).
Conclusions
SBS is associated with increased percent body fat and reduced fat-free mass, suggesting that improved dietary and therapeutic interventions are needed to restore normal metabolic indices and avoid risk of metabolic syndrome in SBS patients.
Similar content being viewed by others
Abbreviations
- SBS:
-
Short bowel syndrome
- PN:
-
Parenteral nutrition
- NHANES:
-
National Health and Nutrition Examination Survey
- DXA:
-
Dual-energy X-ray absorptiometry
- BIA:
-
Bioelectric impedance analysis
- FFM:
-
Fat-free mass
- FM:
-
Fat mass
- %BF:
-
% body fat
- VFM:
-
Visceral adipose fat mass
- VFV:
-
Visceral adipose fat volume
- BMD:
-
Bone mineral density
- NDSR:
-
Nutrition data system research
- VAT:
-
Visceral adipose tissue
References
Billiauws L, Maggiori L, Joly F, Panis Y. Medical and surgical management of short bowel syndrome. J Visc Surg. 2018;155:283–291.
Boutte HJ, Rubin DC. Short bowel syndrome. In: Bardan E, Shaker R, eds. Gastrointestinal Motility Disorders: A Point-of-Care Clinical Guide. Springer; 2018:343–351.
Levin MS, Rubin DC. Intestinal adaptation. In: Langnas AN, Goulet O, Quigley EM, Tappenden KA, eds. Intestinal Failure. Oxford: Blackwell Publishing; 2008:45–56.
Wang Y, Iordanov H, Swietlicki EA, et al. Targeted intestinal overexpression of the immediate early gene tis7 in transgenic mice increases triglyceride absorption and adiposity. J Biol Chem. 2005;280:34764–34775.
Tantemsapya N, Meinzner-Derr J, Erwin CR, Warner BW. Body composition and metabolic changes associated with massive intestinal resection in mice. J Pediatr Surg. 2008;43:14–19.
Barron L, Courtney C, Bao J, et al. Intestinal resection-associated metabolic syndrome. J Pediatr Surg. 2018;53:1142–1147.
Pichler J, Chomtho S, Fewtrell M, Macdonald S, Hill S. Body composition in paediatric intestinal failure patients receiving long-term parenteral nutrition. Arch Dis Child. 2014;99:147–153.
Engelstad HJ, Barron L, Moen J, et al. Remnant small bowel length in pediatric short bowel syndrome and the correlation with intestinal dysbiosis and linear growth. J Am Coll Surg. 2018;227:439–449.
Carlsson E, Bosaeus I, Nordgren S. Body composition in patients with short bowel syndrome: an assessment by bioelectric impedance spectroscopy (BIS) and dual-energy absorptiometry (DXA). Eur J Clin Nutr. 2004;58:853–859.
Carlsson E, Bosaeus I, Nordgren S. Body composition in patients with an ileostomy and inflammatory bowel disease: validation of bio-electric impedance spectroscopy (BIS). Eur J Clin Nutr. 2002;56:680–686.
Tjellesen L, Staun M, Nielsen PK. Body composition changes measured by dual-energy X-ray absorptiometry in patients receiving home parenteral nutrition. Scand J Gastroenterol. 1997;32:686–690.
Skallerup A, Nygaard L, Olesen SS, Kohler M, Vinter-Jensen L, Rasmussen HH. The prevalence of sarcopenia is markedly increased in patients with intestinal failure and associates with several risk factors. Clin Nutr (Edinburgh, Scotland). 2018;37:2029–2035.
Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey, 2018. Available at: https://www.cdc.gov/nchs/nhanes/index.htm. Accessed August 6, 2018.
Li CY, Ford ES, Zhao GX, Balluz LS, Giles WH. Estimates of body composition with dual-energy X-ray absorptiometry in adults. Am J Clin Nutr. 2009;90:1457–1465.
Frivolt K, Hetterich H, Schwerd T, et al. Increase of intra-abdominal adipose tissue in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2017;65:633–638.
Bryant RV, Schultz CG, Ooi S, et al. Visceral adipose tissue is associated with stricturing Crohn’s disease behavior, fecal calprotectin, and quality of life. Inflamm Bowel Dis. 2019;25:592–600.
Marchix J, Goddard G, Helmrath MA. Host-gut microbiota crosstalk in intestinal adaptation. Cell Mol Gastroenterol Hepatol. 2018;6:149–162.
Kliewer KL, Ke JY, Lee HY, et al. Short-term food restriction followed by controlled refeeding promotes gorging behavior, enhances fat deposition, and diminishes insulin sensitivity in mice. J Nutr Biochem. 2015;26:721–728.
Haas V, Kent D, Kohn MR, et al. Incomplete total body protein recovery in adolescent patients with anorexia nervosa. Am J Clin Nutr. 2018;107:303–312.
El Ghoch M, Calugi S, Lamburghini S, Dalle GR. Anorexia nervosa and body fat distribution: a systematic review. Nutrients. 2014;6:3895–3912.
Mayer L, Walsh BT, Pierson RN, et al. Body fat redistribution after weight gain in women with anorexia nervosa. Am J Clin Nutr. 2005;81:1286–1291.
Dulloo AG, Jacquet J, Girardier L. Poststarvation hyperphagia and body fat overshooting in humans: a role for feedback signals from lean and fat tissues. Am J Clin Nutr. 1997;65:717–723.
Shaw K, Gennat H, O’Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006;4:CD003817.
Ohkawara K, Tanaka S, Miyachi M, Ishikawa-Takata K, Tabata I. A dose-response relation between aerobic exercise and visceral fat reduction: systematic review of clinical trials. Int J Obes. 2005;2007:1786–1797.
Verheggen RJ, Maessen MF, Green DJ, Hermus AR, Hopman MT, Thijssen DH. A systematic review and meta-analysis on the effects of exercise training versus hypocaloric diet: distinct effects on body weight and visceral adipose tissue. Obes Rev. 2016;17:664–690.
Chin SH, Kahathuduwa CN, Binks M. Physical activity and obesity: what we know and what we need to know. Obes Rev. 2016;17:1226–1244.
Shook RP. Obesity and energy balance: what is the role of physical activity? Expert Rev Endocrinol Metab. 2016;11:511–520.
Kalaitzakis E, Carlsson E, Josefsson A, Bosaeus I. Quality of life in short-bowel syndrome: impact of fatigue and gastrointestinal symptoms. Scand J Gastroenterol. 2008;43:1057–1065.
Royall D, Greenberg GR, Allard JP, Baker JP, Harrison JE, Jeejeebhoy KN. Critical assessment of body-composition measurements in malnourished subjects with Crohn’s disease: the role of bioelectric impedance analysis. Am J Clin Nutr. 1994;59:325–330.
Tjellesen L, Nielsen PK, Staun M. Body composition by dual-energy X-ray absorptiometry in patients with Crohn’s disease. Scand J Gastroenterol. 1998;33:956–960.
Frank AP, de Souza Santos R, Palmer BF, Clegg DJ. Determinants of body fat distribution in humans may provide insight about obesity-related health risks. J Lipid Res. 2018;60:1710–1719.
Zong G, Zhang Z, Yang Q, Wu H, Hu FB, Sun Q. Total and regional adiposity measured by dual-energy X-ray absorptiometry and mortality in NHANES 1999–2006. Obesity (Silver Spring). 2016;24:2414–2421.
Barreira TV, Staiano AE, Harrington DM, et al. Anthropometric correlates of total body fat, abdominal adiposity, and cardiovascular disease risk factors in a biracial sample of men and women. Mayo Clin Proc. 2012;87:452–460.
Bi X, Seabolt L, Shibao C, et al. DXA-measured visceral adipose tissue predicts impaired glucose tolerance and metabolic syndrome in obese Caucasian and African–American women. Eur J Clin Nutr. 2015;69:329–336.
Rothney MP, Catapano AL, Xia J, et al. Abdominal visceral fat measurement using dual-energy X-ray: association with cardiometabolic risk factors. Obesity (Silver Spring). 2013;21:1798–1802.
Jeppesen PB, Mortensen PB. The influence of a preserved colon on the absorption of medium chain fat in patients with small bowel resection. Gut. 1998;43:478–483.
Pironi L, Joly F, Forbes A, et al. Long-term follow-up of patients on home parenteral nutrition in Europe: implications for intestinal transplantation. Gut. 2011;60:17–25.
Acknowledgments
These studies were supported by NIH NIDDK R01 DK112378 (DCR, NOD, MSL, BWW), NIH NIDDK R01 DK106382 (DCR, MSL), and the Digestive Diseases Research Core Center at Washington University School of Medicine NIDDK P30 DK52574 (NOD, DCR) and the Biobank Core. We thank Kelly Monroe, Latoya Evans, Darren Nix, and Rodney Newberry for their support in the Biobank Core.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Research grant funding was utilized in the conduct of the research including study coordinator staff, body composition analysis, and statistical analysis at Washington University School of Medicine. The authors received no financial assistance with regard to manuscript preparation. All authors have no other financial disclosures.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10620_2019_6032_MOESM1_ESM.tif
Supplemental Figure 1: Correlation of duration of disease, length of remaining small bowel, and duration of PN therapy with body composition. Panels A–C: Correlation of the duration of short bowel syndrome with (A) fat mass, (B) fat-free mass, and (C) visceral fat mass. Panels D–F: Correlation of the length of remaining small bowel versus (D) fat mass, (E) fat-free mass, and (F) visceral fat mass. Panels G–I: Correlation of duration of parenteral nutrition and (G) fat mass, (H) fat-free mass, and (I) visceral fat mass. (TIFF 537 kb)
10620_2019_6032_MOESM2_ESM.tif
Supplemental Figure 2: Correlation of dietary composition (total caloric intake and percentage of calories from fat or carbohydrate) with BMI and fat mass. Panels A–B: Correlation of the percent calories obtained from fat with (A) BMI and (B) fat mass. Panels C–D: Correlation of the percent calories obtained from carbohydrates with (C) BMI and (D) fat mass. Panels E–F: Correlation of total caloric intake versus (E) BMI and (F) fat mass. (TIFF 269 kb)
Rights and permissions
About this article
Cite this article
Chiplunker, A.J., Chen, L., Levin, M.S. et al. Increased Adiposity and Reduced Lean Body Mass in Patients with Short Bowel Syndrome. Dig Dis Sci 65, 3271–3279 (2020). https://doi.org/10.1007/s10620-019-06032-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-06032-4