Abstract
Background
The Suvar, Enhancer of zeste, and Trithorax (SET), myeloid-Nervy-DEAF-1 (MYND) domain-containing protein 3 (SMYD3), was reported to be upregulated in various tumors. However, its role in pancreatic cancer progression remains unclear.
Aims
To explore the role of SMYD3 in the pancreatic cancer.
Methods
The expressions of SMYD3, caspase-3, and matrix metallopeptidase-2 (MMP-2) were detected in pancreatic cancer and non-tumor tissues by immunohistochemistry. The CCK-8 and transwell assays were performed to test proliferation, migration, and invasion ability in short hairpin RNA (shRNA-SMYD3) pancreatic cancer cell line SW1190. RT-PCR and Western blot were used to detect the expressions of SMYD3, caspase-3, and MMP-2 in SW1990 cell line and shRNA-SMYD3 SW1990 cell line.
Results
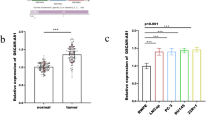
The expressions of SMYD3, caspase-3, and MMP-2 were upregulated in pancreatic cancer. The SMYD3 was positively associated with caspase-3 and MMP-2 expressions in pancreatic cancer tissues. SMYD3, TNM stages, histological differentiation, and lymph node metastasis were identified as an independent prognostic factor. Moreover, interfered SMYD3 expression in SW1990 cell line significantly reduced the cell proliferation, migration, and invasion. RT-PCR and Western blot showed the expression of MMP-2 decreased in shRNA-SMYD3 SW1990 cell line, but no significant change was observed in the caspase-3 expression.
Conclusions
The overexpression of SMYD3 promoted proliferation, migration, and invasion of pancreatic cancer, and SMYD3 may affect the pancreatic cancer progression by regulating MMP-2 rather than caspase-3.








Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30.
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132.
Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–1057.
Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg. 2015;220:530–536.
Suzuki S, Nozawa Y, Tsukamoto S, Kaneko T, Imai H, Minami N. Histone methyltransferase Smyd3 regulates early embryonic lineage commitment in mice. Reproduction. 2015;150:21–30.
Fujii T, Tsunesumi S, Yamaguchi K, Watanabe S, Furukawa Y. Smyd3 is required for the development of cardiac and skeletal muscle in zebrafish. PLoS One. 2011;6:e23491.
Huang L, Xu AM. SET and MYND domain containing protein 3 in cancer. Am J Transl Res. 2017;9:1–14.
Fei X, Ma Y, Liu X, Meng Z. Overexpression of SMYD3 is predictive of unfavorable prognosis in hepatocellular carcinoma. Tohoku J Exp Med. 2017;243:219–226.
Sarris ME, Moulos P, Haroniti A, Giakountis A, Talianidis I. Smyd3 is a transcriptional potentiator of multiple cancer-promoting genes and required for liver and colon cancer development. Cancer Cell. 2016;29:354–366.
Liu H, Liu Y, Kong F, et al. Elevated levels of SET and MYND domain-containing protein 3 are correlated with overexpression of transforming growth factor-β1 in gastric cancer. J Am Coll Surg. 2015;221:579–590.
Tewari M, Quan LT, O’Rourke K, et al. Yama/CPP32ß, a mammalian homologue of CED-3, is a crmA-inhibitable protease that cleaves the death substrate poly ADP-ribose polymerase. Cell. 1995;81:801–809.
Huang KH, Fang WL, Li AF, et al. Caspase-3, a key apoptotic protein, as a prognostic marker in gastric cancer after curative surgery. Int J Surg. 2018;52:258–263.
Efuet ET, Ding XP, Cartwright C, Pan Y, Cohen L, Yang P. Huachansu mediates cell death in non-Hodgkin’s lymphoma by induction of caspase-3 and inhibition of MAP kinase. Int J Oncol. 2015;47:592–600.
Nakagawara A, Nakamura Y, Ikeda H, et al. High levels of expression and nuclear localization of interleukin-1ß converting enzyme (ICE) and CPP32 in favorable human neuroblastomas. Cancer Res. 1997;57:4578–4584.
Satoh K, Kaneko K, Hirota M, Toyota T, Shimosegawa T. The pattern of CPP32/caspase-3 expression reflects the biological behavior of the human pancreatic duct cell tumors. Pancreas. 2000;21:352–357.
Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980;284:67–68.
Stetler-Stevenson WG. The role of matrix metalloproteinases in tumor invasion, metastasis and angiogenesis. Surg Oncol Clin N Am. 2001;10:383–392.
Durlik M, Gardian K. Metalloproteinase 2 and 9 activity in the development of pancreatic cancer. Pol Przegl Chir. 2012;84:377–382.
Liu Y, Liu H, Luo X, Deng J, Pan Y, Liang H. Overexpression of SMYD3 and matrix metalloproteinase-9 are associated with poor prognosis of patients with gastric cancer. Tumour Biol. 2015;36:4377–4386.
Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–1024.
Rajajeyabalachandran G, Kumar S, Murugesan T, et al. Therapeutical potential of deregulated lysine methyltransferase SMYD3 as a safe target for novel anticancer agents. Expert Opin Ther Targets. 2017;21:145–157.
Giakountis A, Moulos P, Sarris ME, Hatzis P, Talianidis I. Smyd3-associated regulatory pathways in cancer. Semin Cancer Biol. 2017;42:70–80.
Paladino D, Yue P, Furuya H, Acoba J, Rosser CJ, Turkson J. A novel nuclear Src and p300 signaling axis controls migratory and invasive behavior in pancreatic cancer. Oncotarget. 2016;7:7253–7267.
Chen H, Yang X, Feng Z, et al. Prognostic value of Caspase-3 expression in cancers of digestive tract: a meta-analysis and systematic review. Int J Clin Exp Med. 2015;8:10225–10234.
Wang HL, Zhou PY, Zhang Y, Liu P. Relationships between abnormal MMP2 expression and prognosis in gastric cancer: a meta-analysis of cohort studies. Cancer Biother Radiopharm. 2014;29:166–172.
Zhang K, Chen X, Zhou J, et al. Association between MMP2-1306 C/T polymorphism and prostate cancer susceptibility: a meta-analysis based on 3906 subjects. Oncotarget. 2017;8:45020–45029.
Ren TN, Wang JS, He YM, Xu CL, Wang SZ, Xi T. Effects of SMYD3 over-expression on cell cycle acceleration and cell proliferation in MDA-MB-231 human breast cancer cells. Med Oncol. 2011;28:S91–S98.
Hamamoto R, Furukawa Y, Morita M, et al. SMYD3 encodes a histone methyltransferase involved in the proliferation of cancer cells. Nat Cell Biol. 2004;6:731–740.
Guil S, Soler M, Portela A, et al. Intronic RNAs mediate EZH2 regulation of epigenetic targets. Nat Struct Mol Biol. 2012;19:664–670.
Brown MA, Foreman K, Harriss J, et al. C-terminal domain of SMYD3 serves as a unique HSP90-regulated motif in oncogenesis. Oncotarget. 2015;6:4005–4019.
Chandramouli B, Silvestri V, Scarno M, Ottini L, Chillemi G. Smyd3 open & closed lock mechanism for substrate recruitment: the hinge motion of C-terminal domain inferred from μ-second molecular dynamics simulations. Biochim Biophys Acta. 2016;1860:1466–1474.
Kim JM, Kim K, Schmidt T, et al. Cooperation between SMYD3 and PC4 drives a distinct transcriptional program in cancer cells. Nucleic Acids Res. 2015;43:8868–8883.
Chen M, Gan X, Yoshino K, et al. Hepatitis C virus NS5A protein interacts with lysine methyltransferase SET and MYND domain-containing 3 and induces activator protein 1 activation. Microbiol Immunol. 2016;60:407–417.
Cock-Rada AM, Medjkane S, Janski N, et al. SMYD3 promotes cancer invasion by epigenetic upregulation of the metalloproteinase MMP-9. Cancer Res. 2012;72:810–820.
Acknowledgment
This study was supported by grants from Anhui science and technology Project (1804h08020277).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, Cl., Huang, Q. Overexpression of the SMYD3 Promotes Proliferation, Migration, and Invasion of Pancreatic Cancer. Dig Dis Sci 65, 489–499 (2020). https://doi.org/10.1007/s10620-019-05797-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-05797-y