Abstract
Background
The benefits of immunosuppressants for sustaining remission and preventing flares of IBD are well known. However, optimal timing for withdrawal has not been determined.
Aims
The objective of this study was to calculate the risk of relapse and predictors after withdrawal of azathioprine (AZA) monotherapy in patients who sustain deep remission.
Methods
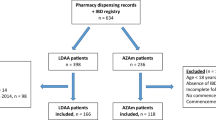
This was a multicenter observational study of patients with IBD in remission whose immunosuppressant had been withdrawn. We recorded demographic variables, disease data, laboratory values, and the results of imaging tests performed at withdrawal and, in patients who relapsed, time to relapse and the efficacy of reintroducing the drug.
Results
Ninety-five patients were included (35 UC and 60 CD). The mean duration of AZA treatment was 87 and 77 months for UC and CD, respectively. Endoscopic remission was evaluated in 23 patients with UC and 35 with CD. After AZA withdrawal, 91% patients with UC and 67% with CD received high doses of salicylates. A total of 26 patients relapsed. The cumulative relapse rate at 5 years was 46% for CD and UC. AZA was reintroduced in 19 patients, of whom 14 responded. Predictors of relapse were corticosteroid dependence, early introduction of AZA (CD), and late introduction of AZA (UC).
Conclusions
Almost half of the patients in whom AZA was withdrawn were in remission at 5 years. The candidates for withdrawal could be better identified based on corticosteroid dependence, previous surgery, timing of initiation, and indication for AZA.



Similar content being viewed by others
References
Harbord M, Eliakim R, Bettenworth D, et al. European Crohn’s and Colitis Organisation [ECCO]. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11:769–784.
Gomollón F, Dignass A, Annese V, et al. ECCO. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11:3–25.
Sultan KS, Berkowitz JC, Khan S. Combination therapy for inflammatory bowel disease. World J Gastrointest Pharmacol Ther. 2017;8:103–113.
Camus M, Seksik P, Bourrier A, et al. Long-term outcome of patients with Crohn’s disease who respond to azathioprine. Clin Gastroenterol Hepatol. 2013;11:389–394.
Kopylov U, Vutcovici M, Kezouh A, Seidman E, Bitton A, Afif W. Risk of lymphoma, colorectal and skin cancer in patients with IBD treated with immunomodulators and biologics: a quebec claims database study. Inflamm Bowel Dis. 2015;21:1847–1853.
Kotlyar DS, Osterman MT, Diamond RH, et al. A systematic review of factors that contribute to hepatosplenic T-cell lymphoma in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011;9:36.e1–41.e1.
Loftus EV Jr. Update on the incidence and prevalence of inflammatory bowel disease in the united states. Gastroenterol Hepatol (N Y). 2016;12:704–707.
Doherty G, Katsanos KH, Burisch J, et al. European Crohn’s and colitis organisation topical review on treatment withdrawal (‘exit strategies’) in inflammatory bowel disease. J Crohns Colitis. 2018;12:17–31.
Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2–6. (Discussion 16-9).
Magro F, Gionchetti P, Eliakim R, et al. European Crohn’s and Colitis Organisation [ECCO]. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and Ileo-anal pouch disorders. J Crohns Colitis. 2017;11:649–670.
Pittet V, Froehlich F, Maillard MH, et al. EPACT-II Update Panellists. When do we dare to stop biological or immunomodulatory therapy for Crohn’s disease? Results of a multidisciplinary European expert panel. J Crohns Colitis. 2013;7:820–826.
Pariente B, Laharie D. Review article: why, when and how to de-escalate therapy in inflammatory bowel diseases. Aliment Pharmacol Ther. 2014;40:338–353.
Moreno-Rincón E, Benítez JM, Serrano-Ruiz FJ, et al. Prognosis of patients with ulcerative colitis in sustained remission after thiopurines withdrawal. Inflamm Bowel Dis. 2015;21:1564–1571.
Cassinotti A, Actis GC, Duca P, et al. Maintenance treatment with azathioprine in ulcerative colitis: outcome and predictive factors after drug withdrawal. Am J Gastroenterol. 2009;104:2760–2776.
Munkholm P, Langholz E, Davidsen M, Bionder V. Frequency of glucocorticoid resistance and dependency in Crohn’s disease. Gut. 1994;35:360–362.
Faubion WA Jr, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121:255–260.
Tung J, Loftus EV Jr, Freese DK, et al. A population-based study of the frequency of corticosteroid resistance and dependence in pediatric patients with Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2006;12:1093–1100.
Cosnes J, Seksik P. Early azathioprine in Crohn’s disease. Inflamm Bowel Dis. 2013;19:674–675.
O’Connor A, Hamlin PJ, Taylor J, Selinger C, Scott N, Ford AC. Postoperative prophylaxis in Crohn’s disease after intestinal resection: a retrospective analysis. Frontline Gastroenterol. 2017;8:203–209.
Regueiro M, Schraut W, Baidoo L, et al. Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology. 2009;136:441.e1–450.e1. (Quiz 716).
Regueiro M, Kip KE, Baidoo L, Swoger JM, Schraut W. Postoperative therapy with infliximab prevents long-term Crohn’s disease recurrence. Clin Gastroenterol Hepatol. 2014;12:1494.e1–1502.e1.
Sorrentino D, Paviotti A, Terrosu G, Avellini C, Geraci M, Zarifi D. Low-dose maintenance therapy with infliximab prevents postsurgical recurrence of Crohn’s disease. Clin Gastroenterol Hepatol. 2010;8:591.e1–599.e1. (Quiz e78-9).
Louis E, Panes J. Adalimumab in ulcerative colitis: can pharmacodynamics be improved based on pharmacokinetics? Gastroenterology. 2012;142:176–178.
Treton X, Bouhnik Y, Mary JY, et al. Groupe D’Etude Thérapeutique Des Affections Inflammatoires Du Tube Digestif (GETAID). Azathioprine withdrawal in patients with Crohn’s disease maintained on prolonged remission: a high risk of relapse. Clin Gastroenterol Hepatol. 2009;7:80–85.
Kennedy NA, Kalla R, Warner B, et al. Thiopurine withdrawal during sustained clinical remission in inflammatory bowel disease: relapse and recapture rates, with predictive factors in 237 patients. Aliment Pharmacol Ther. 2014;40:1313–1323.
Wenzl HH, Primas C, Novacek G, et al. Withdrawal of long-term maintenance treatment with azathioprine tends to increase relapse risk in patients with Crohn’s disease. Dig Dis Sci. 2015;60:1414–1423.
Iborra M, Beltran B, Nos P. Noninvasive testing for mucosal inflammation in inflammatory bowel disease. Gastrointest Endosc Clin N Am. 2016;26:641–656.
Lehmann FS, Burri E, Beglinger C. The role and utility of faecal markers in inflammatory bowel disease. Therap Adv Gastroenterol. 2015;8:23–36.
Hawthorne AB, Logan RF, Hawkey CJ, et al. Randomised controlled trial of azathioprine withdrawal in ulcerative colitis. BMJ. 1992;305:20–22.
Bouhnik Y, Lémann M, Mary JY, et al. Long-term follow-up of patients with Crohn’s disease treated with azathioprine or 6-mercaptopurine. Lancet. 1996;347:215–219.
Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30-year review. Gut. 2002;50:485–489.
Vilien M, Dahlerup JF, Munck LK, Nørregaard P, Grønbaek K, Fallingborg J. Randomized controlled azathioprine withdrawal after more than two years treatment in Crohn’s disease: increased relapse rate the following year. Aliment Pharmacol Ther. 2004;19:1147–1152.
Lémann M, Mary JY, Colombel JF, et al. Groupe D’Etude Thérapeutique des Affections Inflammatoires du Tube Digestif. A randomized, double-blind, controlled withdrawal trial in Crohn’s disease patients in long-term remission on azathioprine. Gastroenterology. 2005;128:1812–1818.
Acknowledgments
We thank María de Miguel Gallo for editorial assistance and Dr Esteban Sáez-González for statistical support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
M Iborra has served as a speaker and consultant from MSD and Takeda. J. Herreras declares no conflict of interest. M. Boscá-Watts reports educational activities, research projects, scientific meetings, and advisory boards sponsored by MSD, Ferring, AbbVie, Janssen, and Takeda. X. Cortés declares no conflict of interest. G. Trejo declares no conflict of interest. E. Cerrillo declares no conflict of interest. D. Hervás declares no conflict of interest. M. Mínguez has served as a speaker, or has received research or education funding from MSD, AbbVie, Takeda, Janssen, and Allergan. B. Beltrán reports educational activities, research projects, scientific meetings, and advisory boards sponsored by MSD, Ferring, Otsuka, and Takeda. P. Nos reports grants and personal fees from MSD, grants from Otsuka, AbbVie, and personal fees from Takeda, Kern, Biogen, and Ferring.
Rights and permissions
About this article
Cite this article
Iborra, M., Herreras, J., Boscá-Watts, M.M. et al. Withdrawal of Azathioprine in Inflammatory Bowel Disease Patients Who Sustain Remission: New Risk Factors for Relapse. Dig Dis Sci 64, 1612–1621 (2019). https://doi.org/10.1007/s10620-018-5429-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-5429-1