Abstract
Background
Ulcerative colitis (UC) is an idiopathic colonic mucosal disease, and its pathogenesis has not been fully understood. Up-frameshift protein 1 (UPF1) is a potential molecule for UC predicted by a computational approach.
Aim
The present study aimed to validate the underlying mechanism of UPF1 in UC.
Methods
UPF1 expression was detected by qRT-PCR, western blotting, and immunohistochemistry in dextran sulfate sodium-induced colitis in mice. To simulate the intestinal inflammation microenvironment, NCM460 human colonic epithelial cells were exposed to a mixture of inflammatory mediators. The potential mechanism involving TNFR1-NF-κB/MAPKs pathway activation was addressed by western blotting, reporter gene assays, and siRNA (siUPF1) or UPF1-expressing plasmid pENTER-transfected cells.
Results
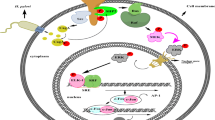
UPF1 was downregulated in colonic epithelial cells of colitic mice, and in vitro, contrary to the mRNA levels of the associated cytokines enhanced in the UPF1 dysregulation group within stimulatory factors, most relevant cytokines were significantly decreased in UPF1 overexpression group. Mechanistically, the increased expression of tumor necrosis factor receptor 1 (TNFR1) was found in NCM460 cells pre-treated with siUPF1, with the activation of IKK/NF-κB and MAPKs pathways, including JNK/AP-1 and P38, but not the ERK1/2 pathway. Moreover, the repression of TNFR1 required the interaction of UPF1 with the promoter.
Conclusion
UPF1, which negatively regulated the transcription of TNFR1, is a novel factor regulating intestinal inflammation. The downregulation of UPF1 activated the TNFR1-dependent NF-κB/MAPKs pathway, and promoting inflammatory responses in colon might act as a causal role in UC.







Similar content being viewed by others
References
Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756–1770.
Atreya R, Neurath MF. IBD pathogenesis in 2014: molecular pathways controlling barrier function in IBD. Nat Rev Gastroenterol Hepatol. 2015;12:67–68.
Zimmerman NP, Vongsa RA, Wendt MK, Dwinell MB. Chemokines and chemokine receptors in mucosal homeostasis at the intestinal epithelial barrier in inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1000–1011.
Zhu H, Wan X, Li J, et al. Computational prediction and validation of BAHD1 as a novel molecule for ulcerative colitis. Sci Rep. 2015;5:12227.
Lee SR, Pratt GA, Martinez FJ, Yeo GW, Lykke-Andersen J. Target discrimination in nonsense-mediated mRNA decay requires Upf1 ATPase activity. Mol Cell. 2015;59:413–425.
Lykke-Andersen S, Jensen TH. Nonsense-mediated mRNA decay: an intricate machinery that shapes transcriptomes. Nat Rev Mol Cell Biol. 2015;16:665–677.
Perlick HA, Medghalchi SM, Spencer FA, Kendzior RJ Jr, Dietz HC. Mammalian orthologues of a yeast regulator of nonsense transcript stability. Proc Natl Acad Sci USA. 1996;93:10928–10932.
Medghalchi SM, Frischmeyer PA, Mendell JT, Kelly AG, Lawler AM, Dietz HC. Rent1, a trans-effector of nonsense-mediated mRNA decay, is essential for mammalian embryonic viability. Hum Mol Genet. 2001;10:99–105.
Wachter A, Hartmann L. NMD: nonsense-mediated defense. Cell Host Microbe. 2014;16:273–275.
Mizoguchi E, Hachiya Y, Kawada M, et al. TNF receptor type I-dependent activation of innate responses to reduce intestinal damage-associated mortality. Gastroenterology. 2008;134:470–480.
Bank S, Skytt Andersen P, Burisch J, et al. Polymorphisms in the inflammatory pathway genes TLR2, TLR4, TLR9, LY96, NFKBIA, NFKB1, TNFA, TNFRSF1A, IL6R, IL10, IL23R, PTPN22, and PPARG are associated with susceptibility of inflammatory bowel disease in a Danish cohort. PLoS ONE. 2014;9:e98815.
Van Hauwermeiren F, Vandenbroucke RE, Grine L, et al. TNFR1-induced lethal inflammation is mediated by goblet and Paneth cell dysfunction. Mucosal Immunol. 2015;8:828–840.
Schrofelbauer B, Hoffmann A. How do pleiotropic kinase hubs mediate specific signaling by TNFR superfamily members? Immunol Rev. 2011;244:29–43.
Zingarelli B, Hake PW, Burroughs TJ, Piraino G, O’Connor M, Denenberg A. Activator protein-1 signalling pathway and apoptosis are modulated by poly(ADP-ribose) polymerase-1 in experimental colitis. Immunology. 2004;113:509–517.
Wirtz S, Popp V, Kindermann M, et al. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc. 2017;12:1295–1309.
McDaniel DK, Eden K, Ringel VM, Allen IC. Emerging roles for noncanonical NF-kappaB signaling in the modulation of inflammatory bowel disease pathobiology. Inflamm Bowel Dis. 2016;22:2265–2279.
Luo C, Zhang H. The role of proinflammatory pathways in the pathogenesis of colitis-associated colorectal cancer. Mediat Inflamm. 2017;2017:5126048.
McGhee JR, Fujihashi K. Inside the mucosal immune system. PLoS Biol. 2012;10:e1001397.
Housley WJ, Fernandez SD, Vera K, et al. Genetic variants associated with autoimmunity drive NFkappaB signaling and responses to inflammatory stimuli. Sci Transl Med. 2015;7:291ra293.
He MM, Smith AS, Oslob JD, et al. Small-molecule inhibition of TNF-alpha. Science. 2005;310:1022–1025.
Chowers Y, Sturm A, Sans M, et al. Report of the ECCO workshop on anti-TNF therapy failures in inflammatory bowel diseases: biological roles and effects of TNF and TNF antagonists. J Crohn’s Colitis. 2010;4:367–376.
Fu SH, Lin MH, Yeh LT, et al. Targeting tumour necrosis factor receptor 1 assembly reverses Th17-mediated colitis through boosting a Th2 response. Gut. 2015;64:765–775.
Schweingruber C, Rufener SC, Zund D, Yamashita A, Muhlemann O. Nonsense-mediated mRNA decay - mechanisms of substrate mRNA recognition and degradation in mammalian cells. Biochim Biophys Acta. 1829;2013:612–623.
Isken O, Maquat LE. The multiple lives of NMD factors: balancing roles in gene and genome regulation. Nat Rev Genet. 2008;9:699–712.
Lu J, Plank TD, Su F, et al. The nonsense-mediated RNA decay pathway is disrupted in inflammatory myofibroblastic tumors. J Clin Invest. 2016;126:3058–3062.
Park OH, Park J, Yu M, An HT, Ko J, Kim YK. Identification and molecular characterization of cellular factors required for glucocorticoid receptor-mediated mRNA decay. Genes Dev. 2016;30:2093–2105.
Johansson ME, Gustafsson JK, Holmen-Larsson J, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63:281–291.
Lissner D, Schumann M, Batra A, et al. Monocyte and M1 macrophage-induced barrier defect contributes to chronic intestinal inflammation in IBD. Inflamm Bowel Dis. 2015;21:1297–1305.
Lawson MM, Thomas AG, Akobeng AK. Tumour necrosis factor alpha blocking agents for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2006;4:CD005112.
Nenci A, Becker C, Wullaert A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561.
Melagraki G, Ntougkos E, Rinotas V, et al. Cheminformatics-aided discovery of small-molecule Protein–Protein Interaction (PPI) dual inhibitors of Tumor Necrosis Factor (TNF) and Receptor Activator of NF-kappaB Ligand (RANKL). PLoS Comput Biol. 2017;13:e1005372.
Dammann K, Khare V, Lang M, et al. PAK1 modulates a PPARgamma/NF-kappaB cascade in intestinal inflammation. Biochim Biophys Acta. 2015;1853:2349–2360.
Gierut JJ, Wood LB, Lau KS, et al. Network-level effects of kinase inhibitors modulate TNF-alpha-induced apoptosis in the intestinal epithelium. Sci Signal. 2015;8:ra129.
Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331.
Tang C, Liang J, Qian J, et al. Opposing role of JNK-p38 kinase and ERK1/2 in hydrogen peroxide-induced oxidative damage of human trophoblast-like JEG-3 cells. Int J Clin Exp Pathol. 2014;7:959–968.
Acknowledgments
The authors would like to thank Prof. Chaohui Yu for critical inputs on the project. The authors would also like to thank Meng Li, Jianghua Chen and the teachers as well as students of the State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, for outstanding technical assistance.
Funding
The present study was financially supported by grants from the National Natural Science Foundation of China (No. 81600413) and Natural Science Foundation of Zhejiang Province (LY16H030005).
Author information
Authors and Affiliations
Contributions
HTZ, SJH and MY initiated the study and performed the experiments, data analysis, and manuscript writing. WGC supervised the study and revised the manuscript. CL, XHL, CXL, GDS, HTC, and XWX contributed to the experimental design, data analysis, and manuscript revision. GQX and LHC had full access to all the data in the present study and had final responsibility for the decision to submit for publication. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that no conflicts of interest.
Ethical approval
The study was approved by the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China. Informed consent was obtained from each subject prior to the study. The maintenance of mice and all animal experimental procedures were conducted in accordance with the National Institutes of Health (NIH) guidelines. All mouse studies were approved by The Animal Care and Use Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University, in accordance with the Chinese guidelines for the care and use of laboratory animals.
Additional information
Huatuo Zhu, Shujun Huang and Min Yue: Co-first authors.
Rights and permissions
About this article
Cite this article
Zhu, H., Huang, S., Yue, M. et al. Dysregulated Up-Frameshift Protein 1 Promotes Ulcerative Colitis Pathogenesis Through the TNFR1-NF-κB/MAPKs Pathway. Dig Dis Sci 63, 2593–2603 (2018). https://doi.org/10.1007/s10620-018-5171-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-5171-8