Abstract
Background and Aims
The pathogenesis of inflammatory bowel disease (IBD) is associated with dysregulation of intestinal immune system. Aryl hydrocarbon receptor (AHR) is believed to control the chronic inflammation in the gut. Besides, interleukin-7 (IL-7) is proved to be an important cytokine that activates mucosal inflammation in IBD. Moreover, intraepithelial lymphocytes (IELs) are one of the key immunological compartments involved in regulating intestinal inflammation. In this study, we investigated the function of 6-formylindolo (3,2-b) carbazole (Ficz), a ligand of AHR, on IL-7, colitis, and IEL phenotypes.
Methods
Colitis was induced by administration of dextran sulfate sodium (DSS) to wild-type C57BL/6J mice for 7 days. Mice were weighted, colon tissues were collected and measured, and histology analyses were performed. IELs were isolated from colon, and the phenotype and activation of IELs were examined using flow cytometry detection. The expression of AHR and IL-7 was measured by immunofluorescence, Western blot, and RT-PCR.
Results
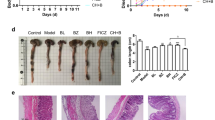
Ficz down-regulated epithelial-derived IL-7 expression in mice with DSS-induced colitis and ameliorated DSS-induced colitis. Ficz also decreased CD8αβ+ and CD8+ IEL subpopulations, enhanced TCRγδ+ IEL subpopulation, and reduced the percentage of activated CD4+ and CD8+ subpopulations.
Conclusions
Ficz could down-regulate epithelial-derived IL-7 expression in mice with DSS-induced colitis and inhibit inflammation in the gastrointestinal tract of mice. AHR-related compounds might be the new and promising therapeutic medicaments for the treatment of patients with IBD.




Similar content being viewed by others
References
Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621.
De Robertis M, Massi E, Poeta ML, et al. The AOM/DSS murine model for the study of colon carcinogenesis: from pathways to diagnosis and therapy studies. J Carcinog. 2011;10:9.
Kanneganti M, Mino-Kenudson M, Mizoguchi E. Animal models of colitis-associated carcinogenesis. J Biomed Biotechnol. 2011;2011:342637.
Melgar S, Karlsson L, Rehnstrom E, et al. Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. Int Immunopharmacol. 2008;8:836–844.
Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol. 2008;21:102–116.
Kewley RJ, Whitelaw ML, Chapman-Smith A. The mammalian basic helix-loop-helix/PAS family of transcriptional regulators. Int J Biochem Cell Biol. 2004;36:189–204.
Kerkvliet NI, Baecher-Steppan L, Shepherd DM, et al. Inhibition of TC-1 cytokine production, effector cytotoxic T lymphocyte development and alloantibody production by 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Immunol. 1996;157:2310–2319.
Kerkvliet NI. Immunological effects of chlorinated dibenzo-p-dioxins. Environ Health Perspect. 1995;103:47–53.
Monteleone I, Rizzo A, Sarra M, et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141:237–248.
Li Y, Innocentin S, Withers DR, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629–640.
Watanabe M, Uen Y, Yajima T, et al. Interleukin 7 is produced by human intestinal epithelial cells and regulates the proliferation of intestinal mucosal lymphocytes. J Clin Invest. 1995;95:2945–2953.
Hara T, Shitara S, Imai K, et al. Identification of IL-7-producing cells in primary and secondary lymphoid organs using IL-7-GFP knock-in mice. J Immunol. 2012;189:1577–1584.
Porter BO, Malek TR. Thymic and intestinal intraepithelial T lymphocyte development are each regulated by the gammac-dependent cytokines IL-2, IL-7, and IL-15. Semin Immunol. 2000;12:465–474.
Carvalho TL, Mota-Santos T, Cumano A, Demengeot J, Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(-/)-mice. J Exp Med. 2001;194:1141–1150.
Yamazaki M, Yajima T, Tanabe M, et al. Mucosal T cells expressing high levels of IL-7 receptor are potential targets for treatment of chronic colitis. J Immunol. 2003;171:1556–1563.
Watanabe M, Yamazaki M, Okamoto R, et al. Therapeutic approaches to chronic intestinal inflammation by specific targeting of mucosal IL-7/IL-7R signal pathway. Curr Drug Targets Inflamm Allergy. 2003;2:119–123.
Dooms Hans. Interleukin-7: Fuel for the autoimmune attack. J Autoimmun. 2013;45:40–48.
Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679.
Bradley LM, Haynes L, Swain SL. IL-7: maintaining T-cell memory and achieving homeostasis. Trends Immunol. 2005;26:172–176.
Yang H, Madison B, Gumucio DL, et al. Specific overexpression of IL-7 in the intestinal mucosa: the role in intestinal intraepithelial lymphocyte development. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1421–G1430.
Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T-cell maintenance. J Immunol. 2005;174:6571–6576.
Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. 2011;11:445–456.
Poussier P, Ning T, Banerjee D, et al. A unique subset of self-specific intraintestinal T cells maintains gut integrity. J Exp Med. 2002;195:1491–1497.
Montufar-Solis D, Garza T, Klein JR. T-cell activation in the intestinal mucosa. Immunol Rev. 2007;215:189–201.
Mosley RL, Klein JR. A rapid method for isolating murine intestine intraepithelial lymphocytes with high yield and purity. J Immunol Methods. 1992;156:19–26.
Zhang L, Ma J, Takeuchi M, et al. Suppression of experimental autoimmune uveoretinitis by inducing differentiation of regulatory T cells via activation of aryl hydrocarbon receptor. Investig Ophthalmol Visual Sci. 2010;51:2109–2117.
Benson JM, Shepherd DM. Aryl hydrocarbon receptor activation by TCDD reduces inflammation associated with Crohn’s disease. Toxicol Sci. 2011;120:68–78.
Schulz VJ, Smit JJ, Willemsen KJ, et al. Activation of the aryl hydrocarbon receptor suppresses sensitization in a mouse peanut allergy model. Toxicol Sci. 2011;123:491–500.
Nohara K, Pan X, Tsukumo S, et al. Constitutively active aryl hydrocarbon receptor expressed specifically in T-lineage cells causes thymus involution and suppresses the immunization-induced increase in splenocytes. J Immunol. 2005;174:2770–2777.
von Freeden-Jeffry U, Davidson N, Wiler R, Fort M, Burdach S, Murray R. IL-7 deficiency prevents development of a non-T cell non-B cell-mediated colitis. J Immunol. 1998;161:5673–5680.
Shinohara T, Nemoto Y, Kanai T, et al. Upregulated IL-7 receptor alpha expression on colitogenic memory CD4þ T cells may participate in the development and persistence of chronic colitis. J Immunol. 2011;186:2623–2632.
Okada E, Yamazaki M, Tanabe M, et al. IL-7 exacerbates chronic colitis with expansion of memory IL-7Rhigh CD4+ mucosal T cells in mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G745–G754.
Watanabe M, Yamazaki M, Okamoto R, et al. Therapeutic approaches to chronic intestinal inflammation by specific targeting of mucosal IL-7/IL-7R signal pathway. Curr Drug Targets Inflamm Allergy. 2003;2:119–123.
Yang H, Madison B, Gumucio DL, et al. Specific overexpression of IL-7 in the intestinal mucosa: the role in intestinal intraepithelial lymphocyte development. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1421–G1430.
Vidali F, Di Sabatino A, Broglia F, et al. Increased CD8+ intraepithelial lymphocyte infiltration and reduced surface area to volume ratio in the duodenum of patients with ulcerative colitis. Scand J Gastroenterol. 2010;45:684–689.
Yang H, Gumucio DL, Teitelbaum DH. Intestinal specific overexpression of interleukin-7 attenuates the alternation of intestinal intraepithelial lymphocytes after total parenteral nutrition administration. Ann Surg. 2008;248:849–856.
Cheroutre H. In IBD eight can come before four. Gastroenterology. 2006;131:667–670.
Tajima M, et al. IL-6-dependent spontaneous proliferation is required for the induction of colitogenic IL-17-producing CD8+ T cells. J Exp Med. 2008;205:1019–1027.
Nancey S, et al. CD8+ cytotoxic T cells induce relapsing colitis in normal mice. Gastroenterology. 2006;131:485–496.
Jabri B, et al. Selective expansion of intraepithelial lymphocytes expressing the HLA-E-specific natural killer receptor CD94 in celiac disease. Gastroenterology. 2000;118:867–879.
Komano H, et al. Homeostatic regulation of intestinal epithelia by intraepithelial γδ T cells. Proc Natl Acad Sci USA. 1995;92:6147–6151.
Guy-Grand D, DiSanto JP, Henchoz P, et al. Small bowel enteropathy: role of intraepithelial lymphocytes and of cytokines (IL-12, IFN-γ, TNF) in the induction of epithelial cell death and renewal. Eur J Immunol. 1998;28:730–744.
Roberts SJ, et al. T-cell αβ+ and γδ+ deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci USA. 1996;93:11774–11779.
Montufar-Solis D, Garza T, Klein JR. T-cell activation in the intestinal mucosa. Immunol Rev. 2007;215:189–201.
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China (NSFC 81330013 and NSFC 81272078 to H.Y., NSFC 81200288 to W.S.W., NSFC 81270451 to W.D.X) and the Program of Changjiang Scholars and Innovative Research (IRT 13050 to HY).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ji, T., Xu, C., Sun, L. et al. Aryl Hydrocarbon Receptor Activation Down-Regulates IL-7 and Reduces Inflammation in a Mouse Model of DSS-Induced Colitis. Dig Dis Sci 60, 1958–1966 (2015). https://doi.org/10.1007/s10620-015-3632-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3632-x