Abstract
Background and Objective
NK4, a competitive antagonist for hepatocyte growth factor (HGF) and the Met receptor, is a bifunctional molecule that acts as an HGF antagonist and an angiogenesis inhibitor. The objective of this study was to investigate the anti-tumor effects of NK4 on the cholangiocarcinoma (CCA) cell line HuCC-T1.
Methods
We assessed the effects of NK4 on proliferation, invasion, migration, and cell cycle progression in mock-transfected HuCC-T1 clones, empty-vector-transfected clones of HuCC-T1 (Hu-Em), and NK4-transfected clones of HuCC-T1 (Hu-NK4), with HuCC-T1 cells serving as the control cells. Correlated with these effects on cellular functions, the mRNA levels of cyclin D1 and cyclin A were monitored using reverse transcription (RT)-PCR and quantitative PCR, and the corresponding protein levels were monitored using Western blotting. In addition, Met phosphorylation and the activity of its important downstream signaling targets protein kinase B (Akt) and glycogen synthase kinase (GSK)-3β were evaluated by Western blotting.
Results
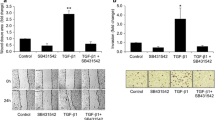
Our data indicate that cell proliferation, invasion, and cell cycle progression of the three types of clones were essentially the same, while these processes were stimulated by HGF in HuCC-T1 and Hu-Em cells, but not in Hu-NK4 cells. Moreover, when stimulated with HGF, the increases in mRNA levels of cyclin D1 and cyclin A were accompanied by corresponding increases in protein levels, and the phosphorylation of Met, Akt, and GSK-3β was upregulated in HuCC-T1 and Hu-Em cells, compared to the levels in the Hu-NK4 cells.
Conclusions
These findings suggest that NK4 gene therapy inhibits HGF/Met-induced growth of human CCA cells by arresting cell cycle progression. It also interferes with Met activation and the downstream phosphatidylinositol-3-kinase/Akt/GSK-3β signaling pathway.






Similar content being viewed by others
References
Mosconi S, Beretta GD, Labianca R, Zampino MG, Gatta G, Heinemann V. Cholangiocarcinoma. Crit Rev Oncol Hematol. 2009;69:259–270.
Menakongka A, Suthiphongchai T. Involvement of PI3K and ERK1/2 pathways in hepatocyte growth factor-induced cholangiocarcinoma cell invasion. World J Gastroenterol. 2010;16:713–722.
Aishima SI, Taguchi KI, Sugimachi K, Shimada M, Tsuneyoshi M. c-erbB-2 and c-Met expression relates to cholangiocarcinogenesis and progression of intrahepatic cholangiocarcinoma. Histopathology. 2002;40:269–278.
Miyamoto M, Ojima H, Iwasaki M, et al. Prognostic significance of overexpression of c-Met oncoprotein in cholangiocarcinoma. Br J Cancer. 2011;105:131–138.
Date K, Matsumoto K, Shimura H, Tanaka M, Nakamura T. HGF/NK4 is a specific antagonist for pleiotrophic actions of hepatocyte growth factor. FEBS Lett. 1997;420:1–6.
Soff GA. Angiostatin and angiostatin-related proteins. Cancer Metastasis Rev. 2000;19:97–107.
Sakai K, Nakamura T, Matsumoto K. Angioinhibitory action of NK4 involves impaired extracellular assembly of fibronectin mediated by perlecan-NK4 association. J Biol Chem. 2009;284:22491–22499.
Nakamura T, Sakai K, Matsumoto K. Anti-cancer approach with NK4: bivalent action and mechanisms. Anticancer Agents Med Chem. 2010;10:36–46.
Matsumoto K, Nakamura T. Mechanisms and significance of bifunctional NK4 in cancer treatment. Biochem Biophys Res Commun. 2005;333:316–327.
Date K, Matsumoto K, Kuba K, Shimura H, Tanaka M, Nakamura T. Inhibition of tumor growth and invasion by a four-kringle antagonist (HGF/NK4) for hepatocyte growth factor. Oncogene. 1998;17:3045–3054.
Kuba K, Matsumoto K, Date K, Shimura H, Tanaka M, Nakamura T. HGF/NK4, a four-kringle antagonist of hepatocyte growth factor, is an angiogenesis inhibitor that suppresses tumor growth and metastasis in mice. Cancer Res. 2000;60:6737–6743.
Boccaccio C, Comoglio PM. Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat Rev Cancer. 2006;6:637–645.
Yue D, Wang Y, Ma P, et al. Effects of transferred NK4 gene on proliferation, migration, invasion and apoptosis of human prostate cancer DU145 cells. Asian J Androl. 2010;12:381–389.
Suzuki Y, Sakai K, Ueki J. Inhibition of Met/HGF receptor and angiogenesis by NK4 leads to suppression of tumor growth and migration in malignant pleural mesothelioma. Int J Cancer. 2010;127:1948–1957.
Johnson DG, Walker CL. Cyclins and cell cycle checkpoints. Annu Rev Pharmacol Toxicol. 1999;39:295–312.
Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36:131–149.
Pardo FS, Su M, Borek C. Cyclin D1 induced apoptosis maintains the integrity of the G1/S checkpoint following ionizing radiation irradiation. Somat Cell Mol Genet. 1996;22:135–144.
Girard F, Strausfeld U, Fernandez A, Lamb NJ. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991;67:1169–1179.
Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501.
Roche S, Downward J, Raynal P, Courtneidge SA. A function for phosphatidylinositol 3-kinase beta (p85alpha-p110beta) in fibroblasts during mitogenesis: requirement for insulin- and lysophosphatidic acid-mediated signal transduction. Mol Cell Biol. 1998;18:7119–7129.
Choudhury GG. Akt serine threonine kinase regulates platelet-derived growth factor-induced DNA synthesis in glomerular mesangial cells: regulation of c-fos AND p27(kip1) gene expression. J Biol Chem. 2001;276:35636–35643.
Gong R, Rifai A, Dworkin LD. Activation of PI3K-Akt-GSK3beta pathway mediates hepatocyte growth factor inhibition of RANTES expression in renal tubular epithelial cells. Biochem Biophys Res Commun. 2005;330:27–33.
Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426.
Acknowledgments
We are grateful to Prof. Toshikazu Nakamura and Prof. Kiyomasa Oka (Osaka University Medical School, Japan) for their generosity in providing the human NK4 expression plasmid pcDNA3/NK4. We also acknowledge the excellent technical assistance of Dr. Yanggang Yuan and Dr. Chenbo Ji (Institute of Pediatrics, Nanjing Medical University, China). This study was supported by grants from the Jiangsu Health Department (H200835) and the Natural Science Foundation of Jiangsu Province (BK2012871).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xianxiu Ge and Youli Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ge, X., Wang, Y., Wang, Y. et al. NK4 Gene Therapy Inhibits HGF/Met-Induced Growth of Human Cholangiocarcinoma Cells. Dig Dis Sci 58, 1636–1643 (2013). https://doi.org/10.1007/s10620-012-2523-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2523-7