Abstract
Background
Gut homeostasis can be altered by the oral administration of health-promoting microorganisms, namely probiotics that are known to reinforce the host immune response.
Aim
The aim of this study was to elucidate the immunomodulatory effect of orally administered probiotic Lactobacillus rhamnosus GG (LGG) in Giardia-infected mice.
Methods
BALB/c mice were fed orally with probiotic LGG either 7 days prior to or simultaneously with the challenge dose of Giardia trophozoites. The administration of the probiotic was continued for 25 days, and immunomodulatory potentials in terms of secretory immunoglobulin A (IgA) levels, CD8+ and CD4+ T lymphocytes, and expression of pro-inflammatory [tumor necrosis factor-alpha, interferon-gamma (INF-γ)] and anti-inflammatory cytokines [interleukin (IL)-4, IL-6, IL-10] were studied.
Results
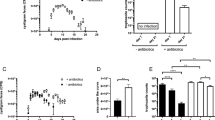
Oral feeding of LGG prior to or simultaneously with the test dose of Giardia seems to have modulated both arms (humoral and cellular) of the mucosal immune system since a significant increase in the levels of specific secretory IgA antibody, IgA+ cells, and CD4+ T lymphocytes were observed in contrast with the decreased percentage of cytotoxic CD8+ T lymphocytes. The stimulated mucosal immune response in probiotic fed Giardia-infected mice was further correlated with the enhanced levels of anti-inflammatory cytokines IL-6 and IL-10 and reduced levels of pro-inflammatory cytokine INF-γ.
Conclusions
This is the first study to show that oral administration of the effective probiotic LGG to Giardia infected mice could be used as a bacterio-therapy that restores the normal gut microflora and modulates the mucosal immune response.










Similar content being viewed by others
References
Ratanapo S, Mungthin M, Soontrapa S. Multiple modes of transmission of giardiasis in primary school children of a rural community, Thailand. Am J Trop Med Hyg. 2008;78:611–615.
Mastronicola D, Giuffre A, Testa F, et al. Giardia intestinalis escapes oxidative stress by colonizing the small intestine: a molecular hypothesis. IUBMB Life. 2011;63:21–25.
Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002;359:564–571.
Lindquist KR, Palm D, Svard SG. Giardia immunity—an update. Trend Parasitol. 2006;22:26–31.
Gillin FD, Reiner DS, McCaffery JM. Cell biology of the primitive eukaryote Giardia lamblia. Annu Rev Microbiol. 1996;50:679–705.
Katelaris P, Seow F, Ngu M. The effect of Giardia lamblia trophozoites on lipolysis in vitro. Parasitology. 1991;103:35–39.
Buret AG. Mechanisms of epithelial dysfunction in giardiasis. Gut. 2007;56:316–317.
Faubert G. Immune response to Giardia duodenalis. Clin Microbiol Rev. 2000;13:35–54.
Stager S, Muller N. Giardia lamblia infections in B-cell-deficient transgenic mice. Infect Immun. 1997;65:3944–3946.
Singer SM, Nash TE. T-cell-dependent control of acute Giardia lamblia infections in mice. Infect Immun. 2000;68:170–175.
Langford TD, Housley MP, Boes M, et al. Central importance of immunoglobulin A in host defense against Giardia spp. Infect Immun. 2002;70:11–18.
Benyacoub J, Perez PF, Rochat F, et al. Enterococcus faecium SF68 enhances the immune response to Giardia intestinalis in mice. J Nutr. 2005;135:1171–1176.
Schiffrin EJ, Brassart D, Servin AL, Rochat F, Don-net-Hughes A. Immune modulation of blood leukocytes in humans by lactic acid bacteria: criteria for strain selection. Am J Clin Nutr. 1997;66:15–20.
FAO/WHO (2001) Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria (October 2001). “Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria”. Food and Agriculture Organization of the United Nations/ World Health Organization, Geneva. Accessed 11 April 2009.
Maldonado Galdeano C, Perdigón G. The probiotic bacteria Lactobacillus casei induces activation of the gut mucosal immune system through the innate immunity. Clin Vaccine Immunol. 2006;13:219–226.
Matsumoto M, Funami K, Tanabe M, et al. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J Immunol. 2003;171:3154–3162.
Kaur P, Bhatia A. Comparison of the immunomodulatory properties of four probiotics strains of Lactobacillus: Prediction for in vivo efficacy. Int J LifeSc Bt Pharm Res. 2012;1:2250–3137.
Shukla G, Devi P, Sehgal R. Effect of Lactobacillus casei as a probiotic on modulation of giardiasis. Dig Dis Sci. 2008;53:2671–2679.
Shukla G, Sharma G, Goyal N. Probiotic characterization of lactobacilli and yeast strains isolated from whey beverage and therapeutic potential of Lactobacillus yoghurt in murine giardiasis. Am J Biomed Sci. 2010;2:248–261.
Shukla G, Sidhu RK. Lactobacillus casei as a probiotic in malnourished Giardia lamblia infected mice: a biochemical and histopathological study. Can J Microbiol. 2011;57:127–135.
Goyal N, Tiwari RP, Shukla G. Lactobacillus rhamnosus GG as an effective probiotic for murine giardiasis. Interdiscip Perspect Infect Dis. 2011;795219. doi:10.1155/2011/795219.
Lowry OH, Rosenbrough JN, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275.
Dunn LA, Upcroft JA, Fowler EV, Matthews BS, Upcroft P. Orally administered Giardia duodenalis extracts enhance an antigen-specific antibody response. Infect Immun. 2001;69:6503–6510.
Nicole L, Brueton MJ. Intraepithelial, lamina propria and Peyer’s patch lymphocytes of the rat small intestine: isolation and characterization in terms of immunoglobulin markers and receptors for monoclonal antibodies. Immunology. 1982;45:775–783.
Erickson KL, Hubbard NE. Probiotic immunomodulation in health and disease. J Nutr. 2000;130:403–409.
Gill HS. Probiotics to enhance anti-infective defenses in the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2003;17:755–773.
Isolauri ES, Yelda K, Pasi A, Heikki A, Seppo S. Probiotic: effects on immunity. Am J Clinical Nut. 2001;73:444S–450S.
Tamboli CP, Caucheteux C, Cortot A, Colombel JF, Desreumaux P. Probiotics in inflammatory Bowel disease. A critical Review. Best Pract Res Clin Gastroenterol. 2003;17:805–820.
Eckmann L. Mucosal defences against Giardia. Parasite Immunol. 2003;25:259–270.
Galdeano CM, de Moreno de Le Blanc A, Vinderola G, Bonet MEB, Perdigón G. Proposed model: mechanisms of immunomodulation induced by probiotic bacteria. Clin Vaccine Immunol. 2007;14:485–492.
Christensen HR, Frokiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol. 2002;168:171–178.
Viljanen M, Kuitunen M, Haahtela T, Juntunen-Backman K, Korpela R, Savilahti E. Probiotic effects on faecal inflammatory markers and on faecal IgA in food allergic atopic eczema/dermatitis syndrome infants. Pediatr Allergy Immunol. 2005;16:65–71.
Malin M, Suomalainen H, Saxelin M, Isolauri E. Promotion of IgA immune response in patients with Crohn’s disease by oral bacteriotherapy with Lactobacillus GG. Ann Nutr Metab. 1996;40:137–145.
Scott KGE, Yu LCH, Buret AG. Role of CD8+ and CD4+ T lymphocytes in jejunal mucosal injury during murine giardiasis. Infect Immun. 2004;72:3536–3542.
Feleszko W, Jaworska J, Rha RD, Steinhausen S, Avagyan A, Jaudszus A. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin Exp Allergy. 2007;37:498–505.
Neurath MF, Weigmann B, Finotto S. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn’s disease. J Exp Med. 2002;195:1129–1143.
Borruel N, Carol M, Casellas F, et al. Increased mucosal tumour necrosis factor alpha production in Crohn’s disease can be downregulated ex vivo by probiotic bacteria. Gut. 2002;51:659–664.
Zhou P, Li E, Zhu N, Robertson J, Nash T, Singer SM. Role of Interleukin-6 in the control of acute and chronic Giardia infection in mice. Infect Immun. 2003;71:1566–1568.
Cebra JC, Periwal SB, Lee G, Lee F, Shroff KE. Development and maintenance of the gut-associated lymphoid tissue (GALT): the role of enteric bacteria and viruses. Dev Immun. 1998;6:13–18.
Acknowledgments
The authors are thankful to Mr. Bhandari, Senior technician, PGIMER, Chandigarh, India for maintaining and providing the Giardia intestinalis culture. The authors are also thankful to Mrs. Bhupinder Singh, Senior technician, PGIMER, for operating the FACS analyzer and to Mr. Gurpreet Singh, technician, PGIMER, for preparing frozen sections of the intestine. The work was funded by the Indian Council of Medical Research, New Delhi, India.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goyal, N., Shukla, G. Probiotic Lactobacillus rhamnosus GG Modulates the Mucosal Immune Response in Giardia intestinalis-Infected BALB/c Mice. Dig Dis Sci 58, 1218–1225 (2013). https://doi.org/10.1007/s10620-012-2503-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2503-y