Abstract
Introduction
MicroRNAs (miRNAs) are a class of small (19–25 nucleotides) noncoding RNAs that regulate the expressions of a wide variety of genes, including some involved in cancer development. Some recent studies show that DNA methylation contributes to down-regulation of microRNA-137 (miR-137) during tumorigenesis. Whether down-regulation of miR-137 also exists in gastric cancer is unknown.
Aim
Our aim was to test the hypothesis that down-regulation of miR-137 also exists in gastric cancer.
Methods
Expression of levels of miR-137 were examined using real-time PCR on paired gastric cancer and adjacent non-cancerous tissues. The methylation status is detected by MSP.
Results
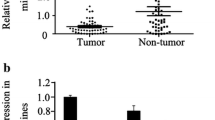
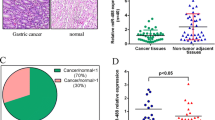
Results show that miR-137 is downregulated by hypermethylation of the promoter in gastric cancer tissues. Epigenetic silencing of miR-137 induced an up-regulation of its targets, Cdc42. Restoration of the miR-137 expression in gastric cancer cell lines downregulated the Cdc42 expression. Restoration of the miR-137 and inactivation of Cdc42 induce apoptosis and cell cycle G1 arrest in gastric cancer cells. Furthermore, the miR-137 expression was found to be inversely correlated with CDC42 expression in gastric caner.
Conclusions
miR-137 is frequently down-regulated in gastric cancer and is a negative regulator of Cdc42.





Similar content being viewed by others
References
Zhang B, Pan X, Cobb GP, Anderson TA. MicroRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12.
Miska EA. How microRNAs control cell division, differentiation and death. Curr Opin Genet Dev. 2005;15:563–568.
Huang YW, Liu JC, Deatherage DE, et al. Epigenetic repression of microRNA-129–2 leads to overexpression of sox4 oncogene in endometrial cancer. Cancer Res. 2009;69:9038–9046.
Dyrskjot L, Ostenfeld MS, Bramsen JB, et al. Genomic profiling of microRNAs in bladder cancer: Mir-129 is associated with poor outcome and promotes cell death in vitro. Cancer Res. 2009;69:4851–4860.
Kozaki K, Imoto I, Mogi S, Omura K, Inazawa J. Exploration of tumor-suppressive microRNAs silenced by DNA hypermethylation in oral cancer. Cancer Res. 2008;68:2094–2105.
Silber J, Lim DA, Petritsch C, et al. Mir-124 and mir-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 2008;6:14.
Bemis LT, Chen R, Amato CM, et al. Microrna-137 targets microphthalmia-associated transcription factor in melanoma cell lines. Cancer Res. 2008;68:1362–1368.
Liu M, Lang N, Qiu M, Xu F, Li Q, Tang Q, Chen J, Chen X, Zhang S, Liu Z, Zhou J, Zhu Y, Deng Y, Zheng Y, Bi F. Mir-137 targets cdc42 expression, induces cell cycle g1 arrest, and inhibits invasion in colorectal cancer cells. Accepted in Int J Cancer.
Balaguer F, Link A, Lozano JJ, Cuatrecasas M, Nagasaka T, Boland CR, Goel A. Epigenetic silencing of mir-137 is an early event in colorectal carcinogenesis. Cancer Res. 2010;70:6609–6618.
Langevin SM, Stone RA, Bunker CH, Grandis JR, Sobol RW, Taioli E. Microrna-137 promoter methylation in oral rinses from patients with squamous cell carcinoma of the head and neck is associated with gender and body mass index. Carcinogenesis. 2010;31:864–870.
Szczur K, Zheng Y, Filippi MD. The small Rho GTPase Cdc42 regulates neutrophil polarity via CD11b integrin signaling. Blood. 2009;114:4527–4537.
Feng Y, Hartig SM, Bechill JE, Blanchard EG, Caudell E, Corey SJ. The cdc42-interacting protein-4 (cip4) gene knock-out mouse reveals delayed and decreased endocytosis. J Biol Chem. 2010;285:4348–4354.
Gomez Del Pulgar T, Valdes-Mora F, Bandres E, et al. Cdc42 is highly expressed in colorectal adenocarcinoma and downregulates ID4 through an epigenetic mechanism. Int J Oncol. 2008;33:185–193.
Sahai E, Marshall CJ. Rho-GTPases and cancer. Nat Rev Cancer. 2002;2:133–142.
Kamai T, Yamanishi T, Shirataki H, et al. Overexpression of RhoA, Rac1, and Cdc42 GTPases is associated with progression in testicular cancer. Clin Cancer Res. 2004;10:4799–4805.
Yang L, Wang L, Zheng Y. Gene targeting of Cdc42 and Cdc42GAP affirms the critical involvement of Cdc42 in filopodia induction, directed migration, and proliferation in primary mouse embryonic fibroblasts. Mol Biol Cell. 2006;17:4675–4685.
Zugasti O, Rul W, Roux P, et al. Raf-MEK-Erk cascade in anoikis is controlled by Rac1 and Cdc42 via Akt. Mol Cell Biol. 2001;21:6706–6717.
Conflict of interest
The author(s) declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Qingjiang Chen and Xiaobing Chen contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.

10620_2010_1536_MOESM1_ESM.jpg
MKN45 cells were subjected to Western blot analysis using antibodies against Cdc42, the phosphorylated form of ERK1/2, total ERK1/2 and Actin. Actin was used as an internal control. (JPEG 33 kb)

10620_2010_1536_MOESM2_ESM.jpg
The expression of Cdc-42 in gastric cancer cell lines MKN45 treated with or without demethylation agent DAC as determined by Western blot. Actin was used as an internal control. (JPEG 74 kb)
Rights and permissions
About this article
Cite this article
Chen, Q., Chen, X., Zhang, M. et al. miR-137 Is Frequently Down-Regulated in Gastric Cancer and Is a Negative Regulator of Cdc42. Dig Dis Sci 56, 2009–2016 (2011). https://doi.org/10.1007/s10620-010-1536-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-010-1536-3