Abstract
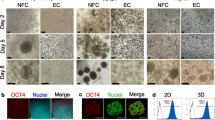
The craving for multiphase materials with adjustable properties for mammalian cell encapsulation persists despite intensive research on 3D cell culture and tissue engineering. This interest is incited by the complex interaction between cells and different materials, various manufacturing methods, cell chip applications, and the aspiration to abolish animal experiments. This study aims to show the feasibility of preparing a stable multiphase material for prolonged mammalian cell embedment and 3D cell culture. The material comprises silica as the solid phase, cell culture medium with serum as the main liquid phase and air as the gas phase. The silica sol-cell culture medium-serum mixture was foamed, and it turned into a stable foamed hydrogel. The stability, flow properties and foaming parameters were studied by rheological and surface tension measurements. The viability of embedded cells was studied by measuring the metabolic activity at different time points. Their sensitivity to the surrounding conditions was compared to cells grown in monolayers by exposing them to a toxic compound. A stable foamed hydrogel with cell culture medium as the main liquid phase was prepared. Based on oscillatory measurements, the foamed hydrogel stays stable for at least 6–7 weeks and the embedded mammalian cells remain viable for the same time period. Appropriate surface tension and viscosity were crucial for an at least twofold volume increase by foaming, which is necessary for the mammalian cells to survive and proliferate. A test with a toxic compound reveals a difference in the sensitivity of cells in monolayer cultures versus embedded cells.



Similar content being viewed by others
References
Amza CG, Zapciu A, Popescu D (2015) Paste extruder—hardware add-on for desktop 3D printers. Technologies 5:50
Antoni D, Burckel H, Josset E, Noel G (2015) Three-dimensional cell culture: a breakthrough in vivo. Int J Mol Sci 16:5517–5527
Au JLS, Yeung BZ, Wientjes MG, Lu Z, Wientjes MG (2016) Delivery of cancer therapeutics to extracellular and intracellular targets: Determinants, barriers, challenges and opportunities. Adv Drug Del Rev 97:280–301
Baeshen MN, Al-Hejin AM, Bora RS, Ahmed MMM, Ramadan HAI, Saini KS, Baeshen NA, Redwan EM (2015) Production of biopharmaceuticals in E. coli: current scenario and future perspectives. J Microbiol Biotechnol 25:953–962
Brinker CJ, Scherer GW (1990) Sol-gel science: the physics and chemistry of sol-gel processing. Academic Press, New York
Cabanas-Polo S, Philippart A, Boccardi E, Hazur J, Boccaccini AR (2016) Facile production of porous bioactive glass scaffolds by the foam replica technique combined with sol–gel/electrophoretic deposition. Cer Int 42:5772–5777
de Soure AM, Fernandes-Platzgummer A, da Silva CL, Cabral JMS (2016) Scalable microcarrier-based manufacturing of mesenchymal stem/stromal cells. J Biotechnol 236:88–109
Edmondson R, Broglie JJ, Adcock AF, Yang L (2014) Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol 12:207–218
Engel BJ, Constantinou PE, Sablatura LK, Doty NJ, Carson DD, Farach-Carson MC, Harrington DA, Zarembinski TI (2015) Multi-layered, hyaluronic acid-based hydrogel formulations suitable for automated 3D high throughput drug screening of cancer-stromal cell co-cultures. Adv Healthc Mater 4:1664–1674
Fernandez-Yague MA, Abbah SA, McNamara L, Zeugolis DI, Pandit A, Biggs MJ (2015) Biomimetic approaches in bone tissue engineering: integrating biological and physicomechanical strategies. Adv Drug Deliv Rev 84:1–29
Gladman AS, Matsumoto EA, Nuzzo RG, Mahadevan L, Lewis JA (2016) Biomimetic 4D printing. Nat Mat 15:413–418
Härmä V, Schukov H-P, Happonen A, Ahonen I, Virtanen J, Siitari H, Åkerfelt M, Lötjönen J, Nees M (2014) Quantification of dynamic morphological drug responses in 3d organotypic cell cultures by automated image analysis. PLoS ONE 9:e96426
Hickman JA, Graeser R, de Hoogt R, Vidic S, Brito C, Gutekunst M, van der Kuip H (2014) Three-dimensional models of cancer for pharmacology and cancer cell biology: capturing tumor complexity in vitro/ex vivo. Biotechnol J 9:1115–1128
Hoppe A, Güldal NS, Boccaccini AR (2011) A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 32:2757–2774
Jones JR, Poologasundarampillai G, Atwood RC, Bernard D, Lee PD (2007) Non-destructive quantitative 3D analysis for the optimisation of tissue scaffolds. Biomaterials 28:1404–1413
Kangasniemi L, Koskinen M, Jokinen M, Toriseva M, Ala-Aho R, Kähäri V-M, Jalonen H, Ylä-Herttuala S, Moilanen H, Stenman U-H, Diaconu I, Kanerva A, Pesonen S, Hakkarainen T, Hemminki A (2009) Extended release of adenovirus from silica implants in vitro and in vivo. Gene Ther 16:103–110
Kortesuo P, Ahola M, Kangas M, Jokinen M, Leino T, Vuorilehto L, Laakso S, Kiesvaara J, Yli-Urpo A, Marvola M (2002) Effect of synthesis parameters of the sol-gel-processed spray-dried silica gel microparticles on the release rate of dexmedetomidine. Biomaterials 23:2795–2801
Krzan M, Caps H, Vandewalle N (2013) High stability of the bovine serum albumine foams evidenced in Hele-Shaw cell. Coll Surf A 438:112–118
Langhans SA (2018) Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front Pharmacol 9:6
Larasati YA, Yoneda-Kato N, Nakamae I, Yokoyama T, Meiyanto E, Kato JY (2018) Curcumin targets multiple enzymes involved in the ROS metabolic pathway to suppress tumor cell growth. Sci Rep 8:2039–2051
Liu Y, Lim J, Teoh S-H (2013) Review: development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnol Adv 3:688–705
Lovitt CJ, Shelper TB, Avery VM (2014) Advanced cell culture techniques for cancer drug discovery. Biology 3:345–367
McLaughlin RL, Newitt DC, Wilmes LJ, Jones EF, Wisner DJ, Kornak J, Proctor E, Joe BN, Hylton NM (2014) High resolution in vivo characterization of apparent diffusion coefficient at the tumor-stromal boundary of breast carcinomas: A pilot study to assess treatment response using proximity-dependent diffusion-weighted imaging. J Magn Reson Imaging. 39:1308–1313
Montanez-Sauri SI, Beebe DJ, Sung KE (2015) Microscale screening systems for 3D cellular microenvironments: platforms, advances, and challenges. Cell Mol Life Sci 72:237–249
Pampaloni F, Reynaud EG, Stelzer EH (2007) The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 8:839–845
Quan Z, Wu A, Keefe M, Qin X, Yu J, Suhr J, Byun J-H, Kim B-S, Chou T-W (2015) Additive manufacturing of multidirectional preforms for composites: opportunities and challenges. Mat Today 18:9
Rimann M, Graf-Hausner U (2012) Synthetic 3D multicellular systems for drug development. Curr Opin Biotechnol 23:803–809
Ryan SL, Baird AM, Vaz G, Urquhart AJ, Senge M, Richard DJ, O’Byrne KJ, Davies AM (2016) Drug discovery approaches utilizing three-dimensional cell culture. Assay Drug Dev Technol 14:19–28
Sepulveda P, Jones JR, Hench LL (2002) Bioactive sol-gel foams for tissue repair. J Biomed Mater Res 59:340–348
Shamir ER, Ewald AJ (2014) Three-dimensional organotypic culture: experimental models of mammalian biology and disease. Nat Rev Mol Cell Biol 15:647–664
Suna T, Jackson S, Haycock JW, MacNeil S (2006) Culture of skin cells in 3D rather than 2D improves their ability to survive exposure to cytotoxic agents. J Biotechnol 122:372–381
Viitala R, Jokinen M, Tuusa S, Rosenholm JB (2005a) Adjustably bioresorbable sol-gel derived SiO2 matrices for release of large biologically active molecules. J Sol–Gel Sci Technol 36:147–156
Viitala R, Jokinen M, Maunu SL, Jalonen H, Rosenholm JB (2005b) Chemical characterization of bioresorbable sol–gel derived SiO2 matrices prepared at protein-compatible pH. J Non-Cryst Sol 351:3225–3234
Warnock JN, Al-Rubeai M (2006) Bioreactor systems for the production of biopharmaceuticals from animal cells. Biotechnol Appl Biochem 45:1–12
Wua ZY, Hill RG, Yue S, Nightingale D, Lee PD, Jones JR (2011) Melt-derived bioactive glass scaffolds produced by a gel-cast foaming technique. Acta Biomater 7:1807–1816
Xynos ID, Edgar AJ, Buttery LDK, Hench LL, Polak JM (2000) Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem Biophys Res Comm 276:461–465
Zhu W, Ma X, Gou M, Mei D, Zhang K, Chen S (2016) 3D printing of functional biomaterials for tissue engineering. Curr Op Biotechnol 40:103–112
Acknowledgments
This research was partly funded by the PWO fund of the Artesis Plantijn University College.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jokinen, M., Pittois, K., van den Akker, S. et al. Multiphase matrix of silica, culture medium and air for 3D mammalian cell culture. Cytotechnology 72, 271–282 (2020). https://doi.org/10.1007/s10616-020-00376-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-020-00376-w