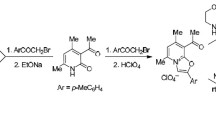
Acyl- and alkyl-derivatives at the N1 and N4 atoms, respectively, of the indoline alkaloids copsinine (1) and pseudocopsinine (2), which were isolated from the plant Vinca erecta, were synthesized. Alkaloids 1 and 2 reacted with alkyl halides to give N4 substitution; with acetic anhydride, N1. In turn, N1-acyl-1 and -2 reacted with alkyl halides to bond the alkyl radical to the N4 position. Reaction products were identified using IR spectroscopy and HPLC-MS. Their structures were elucidated by X-ray crystal structure analyses (XSAs). The tetrahedral hybridization of N1 in the indoline alkaloids was favorable for forming their N1-acetyl derivatives, which is improbable in indole and α-methyleneindoline alkaloids.


Similar content being viewed by others
References
F. S. Sadritdinov and A. G. Kurmukov, Pharmacology of Plant Alkaloids and Their Use in Medicine [in Russian], Meditsina, Tashkent, 1980, pp. 20–78.
A. G. Kurmukov and U. B. Zakirov, Alkaloids and Preparations of Medicinal Herbs for Treating Hypertension [in Russian], Ibn Sino, Tashkent, 1992, pp. 57–60.
Z. Subhan and I. Hindmarch, Eur. J. Clin. Pharm., 28, 567 (1985).
G. Szilagyi, Z. Nagy, L. Balkay, I. Boros, M. Emri, and L. S. Marian, J. Neurol. Sci., 229, 275 (2005).
G. I. David and S. M. Sami, J. Pharm. Sci., 68, 1403 (1979).
Kh. N. Aripov, Chem. Nat. Compd., 13, 624 (1977).
G. V. Lavrenova and V. K. Lavrenov, Encyclopedia of Medicinal Plants [in Russian], Vol. 1, Donetsk, Ukraine, 1997, 84 pp.
Sh. M. Adizov, B. Tashkhodzhaev, R. Zh. Kunafiev, M. M. Mirzaeva, P. P. Upadhyay, and P. Kh. Yuldashev, J. Struct. Chem., 57 (8), 1626 (2016).
Sh. M. Adizov, B. Tashkhodzhaev, R. Zh. Kunafiev, M. M. Mirzaeva, and P. Kh. Yuldashev, J. Struct. Chem., 58 (2), 291 (2017).
Chemist’s Handbook 21. Chemistry and Chemical Engineering, Moscow, 1954, p. 257.
I. I. Grandberg, Organic Chemistry [in Russian], Drofa, Moscow, 2003.
CrysAlisPro. Oxford Diffraction Ltd., Yarnton, England, 2009.
Bruker. APEX2 (Version 2013.6–2), Bruker AXS Inc., Madison, Wisconsin, USA, 2013.
G. M. Sheldrick, Program for Empirical Absorption Correction of Area Detector Data, University of Gottingen, Gottingen, 1996.
G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 64, 112 (2008).
G. M. Sheldrick, Acta Crystallogr., Sect. C: Cryst. Struct. Chem., 71, 3 (2015).
Acknowledgment
The work was sponsored by the Basic Research Program of the Academy of Sciences of the Republic of Uzbekistan, Grant VA-FA-F6-010.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 1, January–February, 2018, pp. 124–128.
Rights and permissions
About this article
Cite this article
Adizov, S.M., Tashkhodzhaev, B., Upadhyay, P.P. et al. Alkyl- and Acyl-Derivatives of Copsinine and Pseudocopsinine and Their Crystal Structures. Chem Nat Compd 54, 147–152 (2018). https://doi.org/10.1007/s10600-018-2278-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-018-2278-2