Humulene and its derivatives (6R)-hydroxy-α-humulene [(6R)-hydroxy-(1E,4E,8E)-4,8,11,11tetramethylcycloundeca-1,4,8-triene], (6R)-acetoxy-α-humulene [(6R)-acetoxy-(1E,4E,8E)-4,8,11,11tetramethylcycloundeca-1,4,8-triene], a coumaric acid ester, 14-hydroxy-α-humulene [14-coumaroxy(1E,4E,8E)-4,8,11,11-tetramethylcycloundeca-1,4,8-triene], (1E,6R,8E)-4,5-epoxy-6-hydroxy-4,8,11,11tetramethylcycloundeca-1,8-diene, and (6R,9S)-4,11,11-trimethyl-8-methylene-1,4-cycloundecadien-6,9-diol were observed in the hydrocarbon extract of Betula pendula (Betulaceae) buds. The GC retention indices were determined for all identified compounds.
Similar content being viewed by others
Caryophyllene and its derivatives were found previously as the principal sesquiterpenoids in birch buds [1–3]. In continuation of our research, rechromatography of the fractions obtained by separation of the petroleum-ether extract [1, 2] isolated several sesquiterpenoids 1–6. Tables 1 and 2 present their spectral properties. Interpretation of the spectral data in the tables and a comparison of them with the literature [4–7] identified 1 as humulene and established the structures of 2–6.

The PMR spectrum of 2 showed resonances for protons and methyls on C atoms of olefinic and tri-substituted double bonds C-1–C-2 and C-8–C-9 that were analogous to those for these bonds in humulene (5.57 ppm, ddd, 1H on C-2; 5.14, d, 1H on C-1; and 4.93, dd, 1H on C-9) and a singlet at 1.44 ppm (3H) for the protons of the methyl on the C atom of the trisubstituted double bond (Table 1).
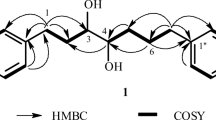
The 13C NMR spectrum (Table 2) contained the corresponding resonances for C atoms of one olefinic (141.4 ppm, d; 126.4, d; C-1 and C-2) and a tri-substituted double bond (131.7, s; 127.5, d; C-8 and C-9). The PMR spectrum showed a singlet at 1.68 ppm for the protons of another methyl that was bonded an unsaturated C atom and a doublet at 4.99 ppm for a single proton of a tri-substituted double bond. Such a splitting pattern occurred because of the adjacent (double resonance) CH–OH group, the presence of which was confirmed by the presence of a secondary C atom (66.8 ppm, 13C NMR DEPT), the resonance of which was displaced to the region characteristic of a C atom bonded to an alcoholic O atom. The compound was further identified after recording 2D 13C–H and NOESY spectra. The spectral results enabled the sequence of C atoms in the compound skeleton to be found as C(CH3)=CH–CH(OH)–CH2–, CH=CH–CH2, and C(CH3)=CH–CH2 and to propose that the isolated compound was 6-hydroxy-α-humulene. The absolute configuration of (6R)-hydroxy-α-humulene [(1E,4E,8E,6R)6-hydroxy-4,8,11,11-tetramethylcycloundeca-1,4,8-triene] was established using a NOESY experiment (Table 1).
J/Hz: for (1): 1-2 = 15.8; 2-3a = 7.3; 2-3b = 10.4; 3a-3b = 13.6; 5-6a = 6.9; 5-6b = 6.3; 9-10a = 7.5; 9-10b = 7.6; 10a-10b = 12.5, 14-5 = 1.2, 15-9 = 0.9; for (2): 1-2 = 16.0; 3a-2 = 8.4; 3b-2 = 6.5; 3a-3b = 13.8; 5-6 = 9.7; 6-7b = 5.0; 6-7a = 8.4; 7a,7b = 12.3; 9-10a = 7.5; 9-10b = 7.6; 10a-10b = 12.5; for (3): 1-2 =15.9; 3a-2 = 8.3; 3b-2 = 6.6; 3a-3b = 14.0; 5-6 = 9.6; 6-7b = 5.2; 6-7a = 8.4; 7a-7b = 12.3; 9-10a = 4.0; 9-10b = 8.6; 10a-10b = 13.0; for (5): 1-2 = 15.8; 2-3a = 6.1; 2-3b = 10.4; 3a-3b = 13.4; 5-6 = 9.2; 6-7a = 8.0; 6-7b = 5.0; 7a-7b = 10.1; 9-10a = 7.0; 9-10b = 7.0; 10a-10b = 10.0; for (6): 1-2 = 16.2; 2-3a = 8.5; 2-3b = 5.7; 5-6 = 9.5; 6-7a = 4.8; 6-7b = 9.5; 7a-7b = 12.8; 9-10a = 3.9; 9-10b = 3.7; 10a-10b = 14.1.
The presence of acetyl derivative 3 with retention index 1789 in fraction 4 (neutral compounds) [1] was confirmed after acetylation of 2 and comparison of the chromatographic and spectrometric (mass spectrum) properties of the alcohol-acetate fraction components. Free alcohol 2 was also detected (GC–MS) in the hydrocarbon extract of buds [1]. The isolation of both (6R)-acetoxy-α-humulene [(1E,4E,8E,6R)-6-acetoxy-4,8,11,11-tetramethylcycloundeca-1,4,8-triene] (3) and (6R)hydroxy-α-humulene (2) from natural sources has apparently not been reported. Earlier 10-acetoxy-α-humulene was isolated from Seseli vayredanum [8] and 14-acetoxy-α-humulene from juniper wood [4].
14-Hydroxy-α-humulene was obtained from the fraction of the hydrocarbon extract [1] containing esters (band at 1720 cm–1 in the IR spectrum). The PMR spectrum of the fraction suggested that aromatic (weak-field resonances at 6– 8 ppm) and aliphatic (strong-field resonances at 0.9–5.5 ppm) groups were present. The saponification products were cis- and trans-coumaric acids and four sesquiterpene alcohols that were separated by chromatography over silica gel. The saponification did not cause isomerization because the PMR spectrum of the initial ester fraction had the same ratio of H atoms on double-bonded C atoms in the aliphatic region of trans- (δ 7.64 ppm, d, J = 16.0 Hz and 6.39, d, J = 16.0) and cis-coumaric acid (7.09, d, J = 12.6 and 5.94, d, J = 12.6).
Compounds with retention indices 1670, 1702, and 1666 were identified by comparison (TLC, GC–MS) with those isolated by us earlier [3] as 6-hydroxy-β-caryophyllene, 14-hydroxy-β-caryophyllene, and (1S,4R,8R)-9,9-dimethyl-2,5dimethylenebicyclo[6.2.0]decan-4-yl)methanol. A compound with retention index 1746 was not identified from the mass spectrum. The spectral properties (Tables 1 and 2) of the compound were consistent with those for 14-hydroxy-β-humulene [(1E,4E,8E)-8,11,11-trimethylcycloundeca-1,4,8-trienyl-4-methanol], which was identified earlier in essential oil of juniper wood [4] and was isolated from Inula roots [9]. Thus, 14-hydroxy-α-humulene is the alcohol constituent of the esters of trans(4a) and cis- (4b) coumaric acids. The isolation of 14-hydroxy-α-humulene from any parts of Betula trees has apparently not been reported. Coumarates of 14-hydroxy-α-humulene (4a and 4b) were isolated for the first time from plant raw material.
Compound 5 was isolated by rechromatography of the neutral compounds [1] containing sesquiterpene alcohols. The following alcohols were identified in the order of elution from silica gel as 6-hydroxy-β-caryophyllene, 6-hydroxy-α-humulene (2), 14-hydroxy-β-caryophyllene, α-betulenol, and [(1S,4R,8R)-9,9-dimethyl-2,5-dimethylenebicyclo[6.2.0]decan4-yl]methanol.
Oxides of sesquiterpene alcohols eluted from the column immediately after the alcohols. These were (4R,5S)-epoxy(6R)-hydroxy-β-caryophyll-8(15)-ene, (4S,5S)-epoxy-(6R)-hydroxy-β-caryophyll-8(15)-ene, compound 5, (4S,5S)-epoxy-14-hydroxy-β-caryophyll-8(15)-ene, and (4R,5S)-epoxy-14-hydroxy-β-carylphyll-8(15)-ene. The PMR spectrum of 5 (Table 1) contained resonances characteristic of humulene and its derivatives including a doublet for the C-1 proton that was centered at 5.23 ppm, a multiplet at 5.26 for the proton of the neighboring C atom (double resonance), a triplet at 5.03 for the C-9 proton, a resonance for protons of the methyl bound to the unsaturated C atom (1.63, s, 3H), and a resonance for the methyl on the C atom with oxygen functional group at 1.31. Similar resonances were observed in humulene epoxide that was isolated previously [10]. However, there was a difference in the position of the resonance for the C-5 proton.
The C-5 proton resonated in the PMR spectrum of the compound isolated by us as a doublet at 2.65 ppm and not as a doublet of doublets at 2.52 ppm [10]. The proton showed coupling (double resonance) with the proton (multiplet at 3.67) on the C atom of the secondary alcohol. It was assumed that the isolated compound was 6-hydroxy-α-humulene 4,5-epoxide (5). The large spin–spin coupling constant (9.2 Hz) between th protons on C-5 and C-6 suggested that they were positioned oppositely relative to the ring plane. Because humulene epoxide is most likely formed from (6R)-hydroxy-α-humulene, the hydroxyl of which is directed toward the observer, the compound probably is (1E,8E)-4,5-epoxy-(6R)-hydroxy-4,8,11,11tetramethylcycloundeca-1,8-diene. The isolation of 5 from plant raw material has apparently not been reported.
Compound 6 was isolated by rechromatography over silica gel of the hydrocarbon extract fractions [1] containing sesquiterpene diols. Two compounds that eluted before 6 were identified as (1R,5R,6R,9S)-5,6-dihydroxy-11,11-dimethyl4,8-dimethylenebicyclo[7.2.0]undecane and (1R,5R,6R,9S)-5,6-dihydroxy-11,11-dimethyl-8-methylenebicyclo[7.2.0]undec3-ene.
The PMR spectrum of 6 had all attributes of a humulene derivative. There were a doublet for the C-1 proton centered at 5.37 ppm with J = 16.2 Hz, a characteristic multiplet centered at 5.71 for the C-2 proton, a resonance for the methyl protons bound to the C atom of the double bond, a singlet centered at 1.71, and resonances for protons of the two other methyls (Table 1). The spectrum showed resonances for protons of an exo-methylene as broad singlets (1H each) centered at 5.15 and 4.96.
A compound with molecular weight (MW) 236 had two hydroxyls. The 13C NMR spectrum exhibited resonances for C atoms of secondary (DEPT) alcohols at 70.6 and 72.6 ppm. The PMR spectrum showed resonances for protons in the region corresponding to protons on an alcoholic C atom. The proton resonating at 4.45 coupled with a proton on an unsaturated C atom (double resonance). This indicated that a –C(CH3)=CH–HC(OH) moiety was present. Because of the complicated nature of the resonance at 4.45, i.e., the proton coupled with other neighboring protons, the only possible site for the secondary hydroxyl was C-6.
The resonance at 3.88 for the proton of the other alcoholic C atom was also shifted to weak field and corresponded to a proton located next to an exo-methylene. This resonance corresponded to the C-9 proton whereas the exo-methylene was situated on C-8. These data were confirmed by assigning resonances using 2D spectroscopy (COSY, COLOC). The stereochemistry of humulene-diol was (1E,6R,9S)-4,11,11-trimethyl-8-methylene-6,9-dihydroxycycloundeca-1,4-diene or 6α,9β-dihydroxyhumulene as established using a NOESY experiment (Table 1). The isolation of (1E,9S,5R)- and (1E,9R,5S)-11,11-dimethyl-4,8-dimethylene-5,9-dihydroxycycloundecanes was reported earlier [11]. The isolation of 6 from plant raw material has apparently not been reported.
Table 3 presents results from quantitative analyses of the isolated humulene and its derivatives and their retention indices.
Experimental
Birch buds (Betula pendula, Betulaceae) were collected immediately before opening in the middle of April from birch growing near the village Roshchino in Leningrad Oblast. The birch species was determined at the Department of Botany, Forestry Academy. Fresh whole birch buds (BB, average moisture 34%) were extracted in a Soxhlet apparatus by petroleum ether (PE) (40–70°C) for 6 h. The extract was evaporated at reduced pressure (26.6 g, 28.8% of dry bud mass), dissolved in Et2O, and separated by the literature method [12] into neutral compounds (21.3 g) and higher fatty acids (5.3 g). Neutral compounds were chromatographed over a column of ASKG silica gel (100–200 mesh, Voskresensk) [1]. The eluent was petroleum ether with 3–8% Et2O and then benzene with an increasing amount (1–20%) of acetone (Table 3).
Analysis was performed using a GC–MS consisting of a 6850A gas chromatograph with a model G2629A control module and a model G2577A Agilent Technologies, Inc., HP5973 Network mass-selective detector. The ionization energy was 70 eV; separator temperature, 280°C; ion source, 230°C. We used an HP-5MS quartz column (30 m × 0.25 mm) with a stationary phase (5% phenylmethylsiloxane) 0.25-μm thick. The analysis was performed with temperature programmed from 100 to 280°C at 5°C/min and isothermal at 280°C for 10 min. The vaporizer temperature was 280°C. The carrier gas (He) flow rate was 1 cm3/min. The sample volume was 0.1 μL. The retention indices of compounds were determined from retention times of n-alkanes (Aldrich). The alkanes were selected such that the retention times of the characterized compounds fell between those of the alkanes. Retention indices were calculated after determining the coefficients of the function I = aτ2 + bτ + c, where I is the retention index and τ, the retention time. The Advanced Grapher 2.08 program was used for the calculation.
TLC was performed on aluminum plates with deposited silica gel (Silufol, Lachema). Sesquiterpenoids were detected using H2SO4 solution (10%) in EtOH.
High-resolution mass spectrometric analysis was carried out in a Varian 902-MS MALDI Mass Spectrometer ion-cyclotron instrument with a 9.4 Tesla superconducting magnet. Samples were desorbed and ionized using the third harmonic of a Nd:YAG laser (355 nm). Dry sample powder was mixed with a significant excess of matrix (2,5-dihydroxybenzoic acid), also as a dry powder. The sample–matrix mixture was dissolved in acetone (matrix partially dissolved). The resulting solution (0.5 μL) was placed on the target and dried in air at room temperature. Accurate molecular weights were determined using an internal calibrant. Weights of standards were recorded simultaneously with those of the studied samples.
PMR spectroscopy used a Bruker AM 500 (500 and 125.76 MHz for 1H and 13C, respectively) instrument, CDCl3 solvent, and the δ-scale. IR spectroscopy was performed on an FSM 1201 IR-Fourier spectrometer (OOO Monitoring). Spectra were taken from films. Specific rotation was determined on a Perkin–Elmer Model 341 polarimeter at wavelength 589 nm using CHCl3 solvent and a 1-dm cuvette.
α-Humulene (4,11,11-trimethyl-1,4,8-cycloundecatriene) (1), oil. One of the first fractions from separation of the PE extract of BB [1] consisted according to GC–MS of two compounds with MW 204. They were separated over a column of silica gel impregnated with AgNO3 (3%) using gradient elution by PE with Et2O added up to 5%. β-Caryophyllene eluted first from the column. A compound (oil) that was identified by PMR (Table 2), 13C NMR, and mass spectra as α-humulene followed it.
Mass spectrum (EI, 70 eV, m/z, I rel, %): 204 (10), 189 (4), 174 (1), 161 (5), 147 (25), 131 (7), 121 (33), 105 (18), 93 (100), 80 (32), 67 (14), 53 (12), 41 (19). IR spectrum (film, ν, cm–1): 1448, 1375, 1008, 885.
(6R)-Hydroxy-α-humulene [(6R)-4,11,11-trimethyl-1,4,8-cycloundecatrienol] (2), oil. The ester fraction (0.5 g) containing acetates (IR spectrum 1720, 1240 cm–1) was analyzed beforehand by GC–MS. Compounds with retention indices 1771 and 1807 were identified as 6-acetoxy-β-caryophyllene and 14-acetoxy-β-caryophyllene. A compound with retention index 1789 was not identified by mass spectroscopy. The fraction was hydrolyzed by refluxing in KOH solution (100 mL, 0.5N) in EtOH for 1.5 h. The saponification products containing sesquiterpene alcohols were separated into three fractions over a column of silica gel with AgNO3 (3%) with elution by PE with Et2O added from 2 to 30%. The fraction that eluted after 6-hydroxy-β-caryophyllene by PE with 25% Et2O contained 2 (NMR spectra, Tables 1 and 2).
Mass spectrum (EI, 70 eV, m/z, I rel, %): 220 (2), 202 (23) [M – H2O]+, 187 (13), 159 (31), 147 (30), 146 (17), 145 (35), 134 (24), 133 (42), 131 (47), 129 (16), 123 (18), 122 (22), 121 (30), 119 (73), 117 (34), 105 (100).
Mass spectrum (MALDI, %): C15H24O + Na; found: 243.1710 (100), 244.1745 (15.8), 245.1785 (1.1); calcd 243.1710 (100), 244.1745 (15.8), 245.1785 (1.1). IR spectrum (film, ν, cm–1): 3358, 1446, 1388, 1362, 1006, 968, 825. [α] 20D +69.8° (c 2.495, CHCl3).
(6R)-Acetoxy-α-humulene [(6R)-acetoxy-4,11,11-trimethyl-1,4,8-cycloundecatriene] (3). Compound 2 (50 mg) was acetylated by a mixture of Py (1.5 mL) and acetic anhydride (1.5 mL) for 24 h at room temperature. The product was an oil consisting of 3. Table 1 lists the PMR spectrum.
Mass spectrum (EI, 70 eV, m/z, I rel, %): 262 (1), 247 (<1), 220 (3), 202 (65), 187 (27), 173 (6), 159 (46), 146 (28), 145 (28), 134 (87), 119 (93), 109 (63), 107 (55), 94 (43), 80 (100), 69 (45), 55 (26), 43 (92). IR spectrum (film, ν, cm–1): 1660, 1530, 1383, 1362, 1109, 1008, 968.
Compound 3 had retention index 1789 according to GC–MS and a mass spectrum analogous to that of the compound from the ester fraction of neutral compounds.
14-p-Coumaroyl-α-humulene (4). The fraction was acetylated and separated by repeated gradient elution using PE:(1–15%)Et2O from a silica-gel column (9:1 by mass) into two parts [TLC using PE:Et2O (10%), R f 0.5 and 0.6, respectively]. Each part was saponified. trans-Coumaric acid was isolated from the larger part; cis-coumaric acid, from the smaller. The ratio of coumarates before saponifation was the same (GC–MS). The retention time of 14-p-coumaroyl-4,11,11-trimethyl-1,4,8-cycloundecatriene (index 3253) by GC–MS was greater than those of the coumarates of 6-hydroxy-β-caryophyllene (index 3082), 14-hydroxy-β-caryophyllene (index 3137), and (1S,4R,8R)-9,9-dimethyl-2,5-dimethylenebicyclo[6.2.0]decan-4-yl methanol (index 3122). GC–MS data were obtained by analyzing the whole coumarate fraction. Ratios of peak areas in chromatograms of the coumarate and alcohol fractions after saponification of the fraction were consistent with the mass spectrum of 14-hydroxy-α-humulene coumarate. The ratio of peak areas did not change after saponification.
Mass spectrum (EI, 70 eV, m/z, Irel, %): 366 (<1), 340 (2), 331 (2), 327 (2), 283 (2), 282 (3), 281 (10), 253 (4), 207 (18), 202 (17), 187 (11), 173 (3), 164 (9), 163 (3), 159 (12), 147 (100), 134 (39), 133 (10), 131 (8), 119 (69), 107 (9), 105 (16), 91 (32), 77 (8), 69 (7), 65 (8), 41 (11).
14-Hydroxy-α-humulene (4,11,11-trimethyl-1,4,8-cycloundecatrien-14-ol), oil. The fraction of neutral compounds of the BB hydrocarbon extract (150 mg) containing the coumarates was hydrolyzed by refluxing in KOH solution (100 mL, 0.5N) in EtOH for 1.5 h. The saponification products, sesquiterpene alcohols (four compounds), were separated by column chromatography over silica gel using benzene with added Et2O (1–2%) into four fractions. Fraction 3 was an oil (22% of total alcohols) containing 14-hydroxy-α-humulene, [α] 20D –13.1° (c 0.96, CHCl3). IR spectrum (film, ν, cm–1): 3355, 3020, 1661, 1018, 971.
6-Hydroxy-α-humulene epoxide [(4,5)-epoxy-(6R)-hydroxy-4,8,11,11-tetramethyl-1,8-cycloundecadiene] (5), oil. The compound was isolated by rechromatography over silica gel from the alcohol fraction of neutral compounds from the BB hydrocarbon extract using gradient elution by PE:acetone (1–40%). Compound 5 was eluted by the last. Table 1 lists the PMR spectrum.
Mass spectrum (EI, 70 eV, m/z, Irel, %): 236 (<1), 221 (4), 207 (1), 203 (1), 190 (1), 175 (1), 168 (7), 163 (2), 154 (6), 139 (11), 138 (12), 135 (7), 121 (14), 109 (100), 93 (24), 82 (74), 67 (60), 55 (23), 43 (50), 29 (9).
6,9-Dihydroxyhumulene [(6R,9S)-4,11,11-trimethyl-8-methylene-1,4-cycloundecadien-6,9-diol] (6), oil. The compound was isolated by rechromatography over silica gel of the alcohol fraction of neutral compounds from the BB hydrocarbon extract using gradient elution by PE:acetone (1–70%). Compound 6 eluted by adding 65–70% acetone to PE. Tables 1 and 2 list the NMR spectra.
Mass spectrum (EI, 70 eV, m/z, I rel, %): 236 (1), 237 (<1), 218 (6), 203 (15), 193 (4), 185 (14), 175 (18), 157 (17), 151 (56), 147 (25), 135 (38), 133 (37), 123 (68), 121 (50), 107 (66), 95 (76), 91 (79), 81 (83), 69 (83), 55 (58), 41 (100), 29 (18). Mass spectrum (MALDI, %): C15H24O2 + Na; found: 259.1661 (100), 260.1692 (15.9), 261.1738 (1.3); calcd: 259.1674 (100), 260.1708 (16.2), 261.1741 (1.2). IR spectrum (film, ν, cm–1): 3390, 1715, 1446, 1385, 1362, 1095, 1006, 890.
Thus, BB extract produced by PE extraction contains α-humulene and its previously known derivatives 6-hydroxyhumulene, 6-acetoxyhumulene, 6α,9β-dihydroxyhumulene, 14-coumaroxyhumulene, and 4,5-epoxy-6-hydroxyhumulene. The GC retention indices of all identified compounds were determined. The structures of the isolated humulene derivatives were established by NMR spectroscopy and mass spectrometry.
References
D. N. Vedernikov and V. I. Roshchin, Khim. Rastit. Syr’ya, No. 3, 69 (2009).
D. N. Vedernikov and V. I. Roshchin, Khim. Rastit. Syr’ya, No. 3, 75 (2009).
D. N. Vedernikov, N. G. Galashkina, and V. I. Roshchin, Rastit. Resur., 3, 84 (2007).
A. F. Barrero and J. E. Oltra, Flavour Fragrance J., 8, 185 (1993).
N. P. Damodaran and S. Dev, Tetrahedron, 24, 4133 (1968).
A. F. Barrero, M. M. Herrador, and P. Arteaga, Phytochemistry, 31, 203 (1992).
R. Randriamiharisoa, E. M. Gaydou, R. Faure, and J. P. Bianchi, Magn. Reson. Chem., 24, 275 (1986).
A. F. Barrero, M. M. Herrador, and P. Arteaga, Phytochemistry, 31, 203 (1992).
F. Bohlmann, W. F. Abraham, and W. S. Sheldrick, Phytochemistry, 19, 869 (1980).
W.-Y. Tsui and G. D. Brown, J. Nat. Prod., 59, 1084 (1996).
S. Takeda, Y. Iimura, K. Tanaka, E. Kurosawa, and T. Suzuki, Chem. Lett., 155 (1990).
V. I. Roshchin, R. A. Baranova, O. A. Belozerskikh, and V. A. Solov’ev, Khim. Drev., No. 4, 56 (1983).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 6, pp. 752–756, November–December, 2010.
Rights and permissions
About this article
Cite this article
Vedernikov, D.N., Roshchin, V.I. Humulene and its derivatives from Betula pendula buds. Chem Nat Compd 46, 886–891 (2011). https://doi.org/10.1007/s10600-011-9775-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-011-9775-x