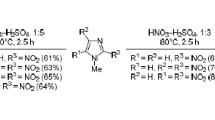
The methylation of 2,4-dinitroimidazole and potassium salt of 2,4,5-trinitroimidazole with dimethyl sulfate was reinvestigated. When the reaction system contained a weak base, the reaction products were 1-methyl-2,4-dinitroimidazole and 1-methyl-2,4,5-trinitroimidazole, respectively. However, in the absence of a base in the reaction system, the products contained oxidation byproducts 1-methylimidazolidine-2,4,5-trione, 1,3-dimethylimidazolidine-2,4,5-trione and recovered starting materials. All products were characterized using FT-IR, NMR, and elemental analysis. The structure of 1-methylimidazolidine-2,4,5-trione and 1,3-dimethylimidazolidine-2,4,5-trione were further confirmed by single crystal X-ray diffraction.

Similar content being viewed by others
References
Nitroimidazoles: Chemistry, Pharmacology and Clinical Applications; Breccia, A.; Cavalleri, B.; Adams, G. E., Eds.; Plenum Press: New York, 1982.
(a) Nair, M. D.; Nagarajan, K. In Prog. Drug Res. 1983, vol. 27, p. 163. (b) Sehgal, R. K.; Webb, M. W.; Agrawal, K. C. J. Med. Chem. 1981, 24, 601. (c) Olender, D.; Żwawiak, J.; Zaprutko, L. J. Heterocycl. Chem. 2010, 47, 1049.
Crozet, M. D.; Rémusat, V.; Curti, C.; Vanelle, P. Synth. Commun. 2006, 36, 3639.
Larina, L.; Lopyrev, V. Nitroazoles: Synthesis, Structure and Applications; Springer: New York, 2009.
(a) Hou, K.; Ma, C.; Liu, Z. New J. Chem. 2013, 37, 2837. (b) Katritzky, A. R.; Yang, H.; Zhang, D.; Kirichenko, K.; Smiglak, M.; Holbrey, J. D.; Reichert, W. M.; Rogers, R. D. New J. Chem. 2006, 30, 349. (c) Lian, P.-B.; Guo, X.-J.; Wang, J.-L.; Chen, L.-Z.; Shen, F.-F. Chem. Heterocycl. Compd. 2018, 54, 1045. [Khim. Geterotsikl. Soedin. 2018, 54, 1045.]
(a) Su, X.; Cheng, X.; Meng, C.; Yuan, X. J. Hazard. Mater. 2009, 161, 551. (b) Su, X.; Cheng, X.; Ge, S. J. Mol. Struct.: THEOCHEM 2009, 895, 44. (c) Jadhav, P. M.; Sarangapani, R.; Ghule, V. D.; Prasanth, H.; Pandey, R. K. J. Mol. Model. 2013, 19, 3027.
Badgujar, D. M.; Talawar, M. B.; Asthana, S. N.; Mahulikar, P. P. J. Hazard. Mater. 2008, 151, 289.
Jadhav, H. S.; Talawar, M. B.; Sivabalan, R.; Dhavale, D. D.; Asthana, S. N.; Krishnamurthy, V. N. J. Hazard. Mater. 2007, 143, 192.
(a) Diao, Y.; Wang, J.-L.; Wang, W.-Y.; Yu, Z.-H.; Liu, L.-L. Chin. J. Explos. Propellants 2012, (2), 40 [In Chinese]. (b) Wang, X.-J.; Cao, D.-L.; Li, Y.-X.; Song, L.; Wang, J.-L. Chin. J. Explos. Propellants 2009, (3), 16 [In Chinese].
(a) Novikov, S. S.; Khmel'nitskii, L. I.; Lebedev, O. V.; Sevast'yanova, V. V.; Epishina, L. V. Chem. Heterocycl. Compd. 1970, 6, 465. [Khim. Geterotsikl. Soedin. 1970, 6, 503.] (b) Novikov, S. S.; Khmel'nitskii, L. I.; Lebedev, O. V.; Epishina, L. V.; Sevost'yanova, V. V. Chem. Heterocycl. Compd. 1970, 6, 614. [Khim. Geterotsikl. Soedin. 1970, 664.] (c) Cho, J. R.; Kim, K. J.; Cho, S. G.; Kim, J. K. J. Heterocycl. Chem. 2002, 39, 141. b Wang, W.; Yang, W.; Ji, Y.-P.; Ding, F. Chin. J. Explos. Propellants 2008(6), 32 [In Chinese].
(a) Ravi, P.; Tewari, S. P. Propellants Explos. Pyrotech. 2012, 37, 544. (b) Duddu, R.; Zhang, M.-X.; Damavarapu, R.; Gelber, N. Synthesis 2011, 17, 2859. (c) Damavarapu, R.; Surapaneni, C. R.; Gelber, N.; Duddu, R. G.; Zhang, M. X.; Dave, P. R. US Patent 7304164 B1. (d) Li, Y.-X.; Song, L.; Ran, J.-P.; Wei, T.-Y. Fine Chem. Intermed. 2009, (3), 65 [In Chinese].
(a) Sudarsanam, V.; Nagarajan, K.; George, T.; Shenoy, S. J.; Iyer, V. V.; Kaulgud, A. P. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 1982, 21B, 1022. (b) Yang, W.; Ji, Y.-P.; Wang, W.; Chen, B. Chin. J. Explos. Propellants 2010, (3), 63 [In Chinese]. (c) Yang, W.; Ji, Y.-P.; Wang, W.; Wang, Y.-L.; Liu, W.-X.; Liu, Y.-J. Fine Chem. Intermed. 2008, (5), 30 [In Chinese].
(a) Zhang, X.-Y.; Chi, Y.; Huang, M.; Wang, J. Chin. J. Energ. Mater. 2012, 20, 685 [In Chinese]. (b) Jerzy, S.; Ewa, S.; Jan, W.; Maria, W. Pol. J. Chem. 1982, 56, 1261.
(a) Gallo, G. G.; Pasqualucci, C. R.; Radaelli, P.; Lancini, G. C. J. Org. Chem. 1964, 29, 862.(b) Stratford, I. J.; Adams, G. E.; Hardy, C.; Hoe, S.; O'Neill, P.; Sheldon, P. W. Int. J. Radiat. Biol. 1984, 46, 731.
Poje, M.; Sokolić-Maravić, L. Tetrahedron 1986, 42, 747.
Lian, P.-B.; Chen, J.; Chen, L.-Z.; Zhao, C.-Y.; Wang, J.-L.; Shen, F.-F. Chem Heterocycl. Compd. 2020, 56, 55. [Khim. Geterotsikl. Soedin. 2020, 56, 55.]
(a) Bulusu, S.; Damavarapu, R.; Autera, J. R.; Behrens, R., Jr.; Minier, L. M.; Villanueva, J.; Jayasuriya, K.; Axenrod, T. J. Phys. Chem. 1995, 99, 5009. (b) Anniyappan, M.; Sonawane, S. H.; Pawar, S. J.; Sikder, A. K. Thermochim. Acta 2015, 614, 93.
Khattab, A. F.; Kappe, T. J. Chem. Res. 2006, 609.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2020, 56(8), 1010–1014
Electronic supplementary material
ESM 1
(PDF 1251 kb)
Rights and permissions
About this article
Cite this article
Lian, PB., Yuan, Y., Chen, J. et al. Study of the methylation reaction of 2,4-dinitroimidazole and potassium 2,4,5-trinitroimidazol-1-ide with dimethyl sulfate. Chem Heterocycl Comp 56, 1010–1014 (2020). https://doi.org/10.1007/s10593-020-02767-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-020-02767-5