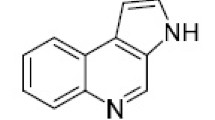
This review article describes methods for the preparation of pyrrolo[1,2-a]quinoxalines, covering literature sources from the last 10 years. The attention was largely paid to new, original methods for the synthesis of pyrrolo[1,2-a]-quinoxalines on the basis of pyrrole and quinoxaline derivatives, as well as multicomponent reactions leading to the formation of both pyrrole and quinoxaline rings.


Similar content being viewed by others
References
Huang, A.; Ma, C. Mini-Rev. Med. Chem. 2013, 13, 607.
(a) Ronga, L.; Del Favero, M.; Cohen, A.; Soum, C.; Le Pape, P.; Savrimoutou, S.; Pinaud, N.; Mullie, C.; Daulouede, S.; Vincendeau, P.; Farvacques, N.; Agnamey, P.; Pagniez, F.; Hutter, S.; Azas, N.; Sonnet, P.; Guillon, J. Eur. J. Med. Chem. 2014, 81, 378. (b) van Heerden, L.; Cloete, T. T.; Breytenbach, J. W.; de Kock, C.; Smith, P.; Breytenbach, J. C.; N'Da, D. D. Eur. J. Med. Chem. 2012, 55, 335. (c) Guillon, J.; Mouray, E.; Moreau, S.; Mullie, C.; Forfar, I.; Desplat, V.; Belisle-Fabre, S.; Pinaud, N.; Ravanello, F.; Le-Naour, A.; Leger, J.-M.; Gosmann, G.; Jarry, C.; Deleris, G.; Sonnet, P.; Grellier, P. Eur. J. Med. Chem. 2011, 46, 2310. (d) Jonet, A.; Guillon, J.; Mullie, C.; Cohen, A.; Bentzinger, G.; Schneider, J.; Taudon, N.; Hutter, S.; Azas, N.; Moreau, S.; Savrimoutou, S.; Agnamey, P.; Dassonville-Klimpt, A.; Sonnet, P. Med. Chem. 2018, 14, 293. (e) Guillon, J.; Cohen, A.; Gueddouda, N. M.; Das, R. N.; Moreau, S.; Ronga, L.; Savrimoutou, S.; Basmaciyan, L.; Monnier, A.; Monget, M.; Rubio, S.; Garnerin, T.; Azas, N.; Mergny, J.-L.; Mullie, C.; Sonnet, P. J. Enzyme Inhib. Med. Chem. 2017, 32, 547.
Xu, H.; Fan, L.-L. Eur. J. Med. Chem. 2011, 46, 1919.
Morelli, E.; Gemma, S.; Budriesi, R.; Campiani, G.; Novellino, E.; Fattorusso, C.; Catalanotti, B.; Coccone, S. S.; Ros, S.; Borrelli, G.; Kumar, V.; Persico, M.; Fiorini, I.; Nacci, V.; Ioan, P.; Chiarini, A.; Hamon, M.; Cagnotto, A.; Mennini, T.; Fracasso, C.; Colovic, M.; Caccia, S.; Butini, S. J. Med. Chem. 2009, 52, 3548.
(a) Guillon, J.; Le Borgne, M.; Rimbault, C.; Moreau, S.; Savrimoutou, S.; Pinaud, N.; Baratin, S.; Marchivie, M.; Roche, S.; Bollacke, A.; Pecci, A.; Alvarez, L.; Desplat, V.; Jose, J. Eur. J. Med. Chem. 2013, 65, 205. (b) Desplat, V.; Moreau, S.; Gay, A.; Fabre, S. B.; Thiolat, D.; Massip, S.; Macky, G.; Godde, F.; Mossalayi, D.; Jarry, C.; Guillon, J. J. Enzyme Inhib. Med. Chem. 2010, 25, 204.
Connell, P.; Lv, W.; Budke, B.; Kozikowski, A. WO Patent 2017149493A1; Сhem. Аbstr. 2017, 167, 348866.
Brindisi, M.; Brogi, S.; Maramai, S.; Grillo, A.; Borrelli, G.; Butini, S.; Novellino, E.; Allara, M.; Ligresti, A.; Campiani, G.; Di Marzo, V.; Gemma, S. RSC. Adv. 2016, 6, 64651.
Sanchez Alonso, P.; Alajarin Ferrandez, R.; Vaquero Lopez, J. J.; Rodriguez Puyol, M.; Griera Merino, M.; Diez Marques, M. L.; Rodriguez Puyol, D. ES Patent 2522717; Сhem. Аbstr. 2016, 164, 314943.
Mashevskaya, I. V.; Kislina, L. V.; Makhmudov, R. R.; Maslivets, A. N. RU Patent 2471798; Сhem. Аbstr. 2013, 158, 131774.
(a) Desplat, V.; Vincenzi, M.; Lucas, R.; Moreau, S.; Savrimoutou, S.; Pinaud, N.; Lesbordes, J.; Peyrilles, E.; Marchivie, M.; Routier, S.; Sonnet, P.; Rossi, F.; Ronga, L.; Guillon, J. Eur. J. Med. Chem. 2016, 113, 214. (b) Desplat, V.; Vincenzi, M.; Lucas, R.; Moreau, S.; Savrimoutou, S.; Rubio, S.; Pinaud, N.; Bigat, D.; Enriquez, E.; Marchivie, M.; Routier, S.; Sonnet, P.; Rossi, F.; Ronga, L.; Guillon, J. ChemMedChem 2017, 12, 940.
Wang, T.; Tang, Y.; Yang, Y.; An, Q.; Sang, Z.; Yang, T.; Liu, P.; Zhang, T.; Deng, Y.; Luo, Y. Bioorg. Med. Chem. Lett. 2018, 28, 2084.
Gemma, S.; Colombo, L.; Forloni, G.; Savini, L.; Fracasso, C.; Caccia, S.; Salmona, M.; Brindisi, M.; Joshi, B. P.; Tripaldi, P.; Giorgi, G.; Taglialatela-Scafati, O.; Novellino, E.; Fiorini, I.; Campiani, G.; Butini, S. Org. Biomol. Chem. 2011, 9, 5137.
(a) Kalinin, A. A.; Mamedov, V. A. Chem. Heterocycl. Compd. 2011, 46, 1423. [Khim. Geterotsikl. Soedin. 2010, 1763.] (b) Mamedov, V. A.; Kalinin, A. A. Chem. Heterocycl. Compd. 2010, 46, 641. [Khim. Geterotsikl. Soedin. 2010, 803.]
He, Z.; Bae, M.; Wu, J.; Jamison, T. F. Angew. Chem., Int. Ed. 2014, 53, 14451.
Zhang, Z.; Li, J.; Zhang, G.; Ma, N.; Liu, Q.; Liu, T. J. Org. Chem. 2015, 80, 6875.
Schulte, A.; Situ, X.; Saito, S.; Wunsch, B. Chirality 2014, 26, 793.
Potikha, L. M.; Kovtunenko, V. A. Chem. Heterocycl. Compd. 2009, 45, 1396. [Khim. Geterotsikl. Soedin. 2009, 1734.]
An, Z.; Zhao, L.; Wu, M.; Ni, J.; Qi, Z.; Yu, G.; Yan, R. Chem. Commun. 2017, 53, 11572.
An, Z.; Jiang, Y.; Guan, X.; Yan, R. Chem. Commun. 2018, 54, 10738.
(a) Zhang, Z.; Xie, C.; Tan, X.; Song, G.; Wen, L.; Gao, H.; Ma, C. Org. Chem. Front. 2015, 2, 942. (b) Mani, G. S.; Subba Rao, A. V.; Tangella, Y.; Sunkari, S.; Sultana, F.; Namballa, H. K.; Shankaraiah, N.; Kamal, A. New J. Chem. 2018, 42, 15820.
Reddy, L. M.; Reddy, V. V.; Putta, C. S.; Satteyyanaidu, V.; Reddy, C. K.; Subba Reddy, B. V. ChemistrySelect 2018, 3, 9881.
Li, J.; Zhang, J.; Yang, H.; Gao, Z.; Jiang, G. J. Org. Chem. 2017, 82, 765.
(a) Ramamohan, M.; Sridhar, R.; Raghavendrarao, K.; Paradesi, N.; Chandrasekhar, K. B.; Jayaprakash, S. Synlett 2015, 1096. (b) Wang, C.; Li, Y.; Guo, R.; Tian, J.; Tao, C.; Cheng, B.; Wang, H.; Zhang, J.; Zhai, H. Asian J. Org. Chem. 2015, 4, 866.
(a) Lade, J. J.; Patil, B. N.; Sathe, P. A.; Vadagaonkar, K. S.; Chetti, P.; Chaskar, A. C. ChemistrySelect 2017, 2, 6811. (b) Lade, J. J.; Patil, B. N.; Vhatkar, M. V.; Vadagaonkar, K. S.; Chaskar, A. C. Asian J. Org. Chem. 2017, 6, 1579. (c) Liu, H.; Zhou, F.; Luo, W.; Chen, Y.; Zhang, C.; Ma, C. Org. Biomol. Chem. 2017, 15, 7157.
Dai, C.; Deng, S.; Zhu, Q.; Tang, X. RSC Adv. 2017, 7, 44132.
(a) Kamal, A.; Babu, K. S.; Kovvuri, J.; Manasa, V.; Ravikumar, A.; Alarifi, A. Tetrahedron Lett. 2015, 56, 7012. (b) Singh, D. K.; Nath, M. Beilstein J. Org. Chem. 2014, 10, 808. (c) Wang, C.; Li, Y.; Zhao, J.; Cheng, B.; Wang, H.; Zhai, H. Tetrahedron Lett. 2016, 57, 3908. (d) Krishna, T.; Reddy, T. N.; Laxminarayana, E.; Kalita, D. ChemistrySelect 2019, 4, 250.
(a) Huo, H.-R.; Tang, X.-Y.; Gong, Y.-F. Synthesis 2018, 2727. (b) Verma, A. K.; Jha, R. R.; Sankar, V. K.; Aggarwal, T.; Singh, R. P.; Chandra, R. Eur. J. Org. Chem. 2011, 6998. (c) Preetam, A.; Nath, M. RSC Adv. 2015, 5, 21843.
(a) Rashidi, R.; Nasr-Esfahani, M.; Mohammadpoor-Baltork, I.; Tangestaninejad, S.; Moghadam, M.; Mirkhani, V. Monatsh. Chem. 2018, 149, 557. (b) Li, Y.; Su, Y.-H.; Dong, D.-J.; Wu, Z.; Tian, S.-K. RSC Adv. 2013, 3, 18275.
Maiti, B.; Sun, Ch.-M. New J. Chem. 2011, 35, 1385.
(a) Liu, G.; Zhou, Y.; Lin, D.; Wang, J.; Zhang, L.; Jiang, H.; Liu, H. ACS Comb. Sci. 2011, 13, 209. (b) Patil, N. P.; Lakshmi, P. G. V. V.; Singh, V. Eur. J. Org. Chem. 2010, 4719. (c) Patil, N. T.; Kavthe, R. D.; Raut, V. S.; Reddy, V. V. J. Org. Chem. 2009, 74, 6315.
Xie, C.; Zhang, Z.; Li, D.; Gong, J.; Han, X.; Liu, X.; Ma, C. J. Org. Chem. 2017, 82, 3491.
Pereira, M. de F.; Thiery, V. Org. Lett. 2012, 14, 4754.
Liu, H.; Duan, T.; Zhang, Z.; Xie, C.; Ma, C. Org. Lett. 2015, 17, 2932.
(a) Li, Z.; Yan, N.; Xie, J.; Liu, P.; Zhang, J.; Dai, B. Chin. J. Chem. 2015, 33, 589. (b) Jiang, Z.; Zhang, J.; Tong, Y.; Shi, X.; Miao, D.; Han, S. Chin. J. Org. Chem. 2014, 34, 1845.
Ammermann, S.; Hrib, Ch.; Jones, P. G.; du Mont, W.-W.; Kowalsky, W.; Johannes, H.-H. Org. Lett. 2012, 14, 5090.
Mamedov, V. A.; Khafizova, E. A.; Zamaletdinova, A. I.; Voronina, J. K.; Kadyrova, S. F.; Mironova, E. V.; Krivolapov, D. B.; Rizvanov, I. Kh.; Sinyashin, O. G. Chem. Heterocycl. Compd. 2017, 53, 560. [Khim. Geterotsikl. Soedin. 2017, 53, 560.]
Mashevskaya, I. V.; Mokrushin, I. G.; Bozdyreva, K. S.; Maslivets, A. N. Russ. J. Org. Chem. 2011, 47, 253. [Zh. Org. Khim. 2011, 47, 261.]
(a) Keivanloo, A.; Kazemi, S. S.; Nasr-Isfahani, H.; Bamoniri, A. Tetrahedron 2016, 72, 6536. (b) Keivanloo, A.; Soozani, A.; Bakherad, M.; Mirzaee, M.; Rudbari, H. A.; Bruno, G. Tetrahedron 2017, 73, 1633.
Wang, M.; Liu, C.; Gu, Y. Tetrahedron 2016, 72, 6854.
(a) Moradi, L.; Piltan, M.; Abasi, G. Helv. Chim. Acta 2014, 97, 646. (b) Sanaeishoar, T.; Adibi-Sedeh, S.; Karimian, S. Comb. Chem. High Throughput Screening 2014, 17, 157.
(a) Piltan, M. J. Chem. Res. 2016, 40, 410. (b) Piltan, M.; Moradi, L.; Abasi, G.; Zarei, S. A. Beilstein J. Org. Chem. 2013, 9, 510.
Piltan, M. Chin. Chem. Lett. 2014, 25, 1507.
(a) Nicolescu, A.; Deleanu, C.; Georgescu, E.; Georgescu, F.; Iurascu, A.-M.; Shova, S.; Filip, P. Tetrahedron Lett. 2013, 54, 1486. (b) Georgescu, E.; Nicolescu, A.; Georgescu, F.; Teodorescu, F.; Shova, S.; Marinoiu, A. T.; Dumitrascu, F.; Deleanu, C. Tetrahedron 2016, 72, 2507. (c) Georgescu, E.; Nicolescu, A.; Georgescu, F.; Shova, S.; Toedorescu, F.; Macsim, A.-M.; Deleanu, C. Synthesis 2015, 1643.
(a) Georgescu, E.; Nicolescu, A.; Georgescu, F.; Teodorescu, F.; Marinescu, D.; Macsim, A.-M.; Deleanu, C. Beilstein J. Org. Chem. 2014, 10, 2377. (b) Moldoveanu, C.; Zbancioc, G.; Mantu, D.; Maftei, D.; Mangalagiu, I. PLoS One 2016, 11, e0156129.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2019, 55(7), 584–597
Rights and permissions
About this article
Cite this article
Kalinin, A.A., Islamova, L.N. & Fazleeva, G.M. New achievements in the synthesis of pyrrolo[1,2-a]quinoxalines. Chem Heterocycl Comp 55, 584–597 (2019). https://doi.org/10.1007/s10593-019-02501-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-019-02501-w