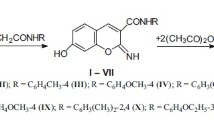
The formyl group of 4H-thieno[3,2-c]chromene-2-carbaldehyde was transformed into the respective nitrile, amide, ester, carboxylic, hydroxamic, or hydroxy group. Electrophilic substitution in 4H-thieno[3,2-c]chromene-2-carbaldehyde was shown to occur at the С-8 atom, while oxidation with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone in methanol led to 4-methoxy-4H-thieno[3,2-c]chromene-2-carbaldehyde. The latter compound was found to possess high antiulcer activity.
Similar content being viewed by others
References
M. I. Hegab and M. M. Abdulla, Arch. Pharm. Chem. Life Sci., 339, 41 (2006).
K. C. Majumdar and A. Biswas, Monatsh. Chem., 135, 1001 (2004).
Y. Makisumi, JP Pat. Appl. 48000597 (1972).
Y. Makisumi, JP Pat. Appl. 48000596 (1973).
V. V. Mulwad and J. M. Shirodkar, Indian J. Heterocycl. Chem., 12, 311 (2003).
M. J. Meegan and D. V. Tyndall, J. Chem. Res., Synop., 239 (1981).
D. F. Rogers, R. W. Godfrey, K. Castro, S. Majumdar, and P. K. Jeffery, Agents Actions, 33, 358 (1991).
S. E. Webber and J. G. Widdicombe, Agents Actions, 24, 65 (1988).
C. G. Rimbault, EP Pat. Appl. 0193493.
M. Taguchi, R. Suzuki, and A. Mikami, WO Pat. Appl. 2006080439.
A. L. Smith, P. E. Brennan, F. F. Demorin, G. Liu, N. A. Paras, and D. M. Retz, WO Pat. Appl. 2006066172.
L. Bao and A. Kimzey, WO Pat. Appl. 2004093803 (2004).
T. D. Mckee, R. K. Suto, T. Tibbitts, and J. Sowadski, WO Pat. Appl. 2004028535.
T. D. Mckee, R. K. Suto, T. Tibbitts, and J. Sowadski, WO Pat. Appl. 03074497.
T. A. Grese, WO Pat. Appl. 9709044.
A. L. Katsiel, A. N. Sharipova, and A. S. Fisyuk, Mendeleev Commun., 18,169 (2008).
A. S. Fisyuk, Yu. P. Bogza, L. V. Belyaeva, and V. B. Belyaev, Chem. Heterocycl. Compd., 48, 1078 (2012). [Khim. Geterotsikl. Soedin., 48, 1160 (2012).]
R. A. Navarro, L. C. Bleye, A. Gonzalez-Ortega, and M. C. S. Ruiz, Heterocycles, 55 (12), 2369 (2001).
B. Chandra Sekhar, D. V. Ramana, and S. R. Ramadas, Sulfur Lett., 9, 271 (1989).
Y.-C. Xu, E. Lebeau, J. W. Gillard, and G. Attardo , Tetrahedron Lett., 34, 3841 (1993).
J. Barbosa, K. G. Carson, M. W. Gardyan, J. P. Healy, Q. Han, R. Mabon, P. Pabba, J. Tarver, Jr., K. M. Terranova, A. Tunoori, and X. Xu, US Pat. Appl. 2012302562.
S. Do, R. Goldsmith, T. Heffron, A. Kolesnikov, S. Staben, A. G. Olivero, M. Siu, D. P. Sutherlin, B.-Y. Zhu, P. Goldsmith, T. Bayliss, A. Folkes, and N. Pegg, US Pat. Appl. 2009247567.
А. N. Mironov (editor), Manual of Preclinical Studies of Medicines [in Russian], Vol. 1, Grif & Co., Moscow (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 12, pp. 1862–1868, December, 2014.
Rights and permissions
About this article
Cite this article
Bogza, Y.P., Katsiel’, A.L., Sharypova, A.N. et al. Synthesis and Biological Activity of 4H-Thieno[3,2-c]Chromene Derivatives. Chem Heterocycl Comp 50, 1712–1718 (2015). https://doi.org/10.1007/s10593-015-1642-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-015-1642-4