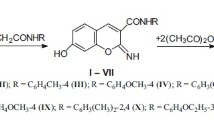
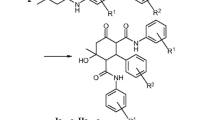
The synthesis and antimicrobial evaluation of a series of chromone-linked substituted heterocyclic derivatives is described. The condensation of 3-formylchromone with acetoacetamide under Knoevenagel–Cope reaction conditions was also explored, and the condensation with 4-hydroxy-6-methyl-2H-pyran-2-one constitutes a facile route to pyranopyrone fused systems. Most of the compounds exhibit good antimicrobial properties.

Similar content being viewed by others

References
G. Sabitha, Aldrichimica Acta, 29, 15 (1996).
M. Kawasei, T. Tanaka, H. Kan, S. Tani, H. Nakashima, and H. Sakagami, In Vivo, 21, 829 (2007).
L. Jund, J. Corse, A. S. King, H. Bayne, and K. Mihrag, Phytochemistry, 10, 2971 (1971).
S. L. El-Ansary, E. I. Aly, and M. A. Halem, J. Pharm. Sci., 33, 379 (1992).
Y. D. Reddy and V. V. Somayojulu, J. Ind. Chem. Soc., 58, 599 (1981).
N. Modranka, J. Nawrot, and E. Graczyk, Eur. J. Med. Chem., 41, 1301 (2006).
O. A. Abd Allah, Farmaco, 55, 641 (2000).
S. S. Parmar and R. Kumar, J. Med. Chem., 11, 635 (1968).
D. A. Horton, G. T. Bourne, and M. L. Smythe, Chem Rev., 103, 893 (2003).
K. Nakano, T. Nakayachi, E. Yasumoto, S. R. Morshed, K. Hashimoto, H. Kikuchi, H. Nishikawa, K. Sugiyama, O. Amano, M. Kawase, and H. Sakagami, Anticancer Res., 24, 711 (2004).
B. Wang, Z. Y. Yang, and T. Li, Bioorg. Med. Chem., 14, 6012 (2006).
B. H. Havsteen, Flavonoids, 96, 67 (2002).
L. Pisco, M. Kordian, K. Peseke, H. Feist, D. Michalik, E. Estrada, J. Carvalho, G. Hamilton, D. Rando, and J. Quincoces, Eur. J. Med. Chem., 41, 401 (2006).
M. M. Dutta, B. N. Goswani, and J. C. S. Kataky, J. Heterocycl. Chem., 23 , 793 (1986).
S. C. Sharma, Bull. Chem. Soc. Jpn., 40, 2422 (1967).
P. Foltinova, M. Lacova, and D. Loos, Farmaco, 55, 21 (2000).
V. N. Chaubey and H. Singh, Bull. Chem. Soc. Jpn., 43, 2233 (1970).
W. O. Foye and P. Tovivich, J. Pharm. Sci., 66, 1607 (1977).
E. B. Akerblom, J. Med. Chem., 17, 609 (1974).
D. Miller, S. Wang, J. Reid, W. Xie, B. Gauvin, M. Kelley, J. Sarup, D. G. Sawutz, M. Miski, R. E. Dolle, and C. R. Faltynek, Drug Dev. Res., 34, 344 (1995).
N. Karali, A. Gursoy, N. Terzioglu, S. Ozkirimli, H. Ozer, and A. C. Ekinci, Arch. Pharm., 331, 254 (1998).
U. Albrecht, M. Lalk, and P. Langer, Bioorg. Med. Chem., 13, 1531 (2005).
E. T. Oganesyan, V. A. Tuskayev, and L. S. Sarkisov, Khim. Farm. Zh., 28, No. 12, 17 (1994).
A. Khodairy, J. Chin. Chem. Soc., 54, 93 (2007).
A. Nohara, H. Kuriki, T. Saijo, K. Ukawa, T. Murata, M. Kanno, and Y. Sanno, J. Med. Chem., 18 , 34 (1975).
R. Gašparová and M. Lácová, Molecules, 10, 937 (2005).
A. Nohara, T. Umetani, and Y. Sanno, Tetrahedron Lett., 22, 1995 (1973).
R. Sagar, P. Singh, R. Kumar, P. R. Maulikb, and A. K. Shaw, Carbohydr. Res., 340, 1287 (2005).
C. K. Ghosh, A. Ray, and A. Patra, J. Heterocycl. Chem., 38, 1459 (2001).
J. W. Lockman, M. D. Reeder, R. Robinson, P. A. Ormonde, D. M. Cimbora, B. L. Williams, and J. A. Willardsen, Bioorg. Med. Chem. Lett., 21, 1724 (2011).
G. N. Rolinson and Elizabeth J. Russell, Antimicrob. Agents Chemother., 2, 51, (1972).
We thank the Research Center, College of Pharmacy and Deanship of Scientific Research, King Saud University for supporting this study. S. W. Ng thanks the Ministry of Higher Education of Malaysia (grant No. UM.C/HIR/MOHE/SC/12) for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, No. 12, pp. 1860-1869, December, 2013.
Rights and permissions
About this article
Cite this article
Bari, A., Ali, S.S., Kadi, A. et al. Synthesis of Some New Heterocyclic Compounds Derived from 3-Formylchromones and Their Antimicrobial Evaluation. Chem Heterocycl Comp 49, 1723–1731 (2014). https://doi.org/10.1007/s10593-014-1424-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-014-1424-4