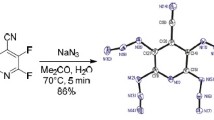
The reactions of 3-cyanotetrafluoropyridine and tetrachloro-3-trifluoromethylpyridine with sodium azide lead to the corresponding 2,4,6-triazido-5-cyano-3-fluoropyridine and 2,4,6-triazido-5-chloro-3-trifluoromethylpyridine. With the aid of comparative X-ray structural analysis of 2,4,6-triazido-5-cyano-3-fluoropyridine and its previously known 3-chloro-substituted derivative it was established that the geometric parameters and reactivity of the azide groups in the asymmetric 2,4,6-triazi-dopyridines depend not only on the position of these groups in the pyridine ring but also on their mutual orientation in space.



Similar content being viewed by others
References
Yu. M. Mikhailov, S. V. Chapyshev, and V. V. Nedel’ko, Russ. Chem. Bull, 58, 2097 (2009).
S. V. Chapyshev, Synlett, 1 (2009).
S. V. Chapyshev, R. Walton, J. A. Sanborn, and P. M. Lahti, J. Am. Chem. Soc., 122, 1580 (2000).
E. Ya. Misochko, A. V. Akimov, and S. V. Chapyshev, J. Chem. Phys., 129, 174510 (2008).
S. V. Chapyshev, D. Grote, C. Finke, and W. Sander, J. Org. Chem., 73, 7045 (2008).
S. V. Chapyshev, P. Neuhaus, D. Grote, and W. Sander, J. Phys. Org. Chem., 23, 340 (2010).
S. D. Moshchitskii, F. F. Zeikan’, A. F. Pavlenko, and V. P. Kukhar’, Khim. Geterotsikl. Soedin., 1492 (1979). [Chem. Heterocycl. Comp., 15, 1197 (1979)].
S. V. Chapyshev, Khim. Geterotsikl. Soedin., 1056 (2001). [Chem. Heterocycl. Comp., 37, 968 (2001)].
S. V. Chapyshev, Khim. Geterotsikl. Soedin., 1650 (1993). [Chem. Heterocycl. Comp., 29, 1426 (1993)].
S. V. Chapyshev and T. Ibata, Heterocycles, 36, 2185 (1993).
S. V. Chapyshev, U. Bergsträsser, and M. Regitz, Khim. Geterotsikl. Soedin., 67 (1996). [Chem. Heterocycl. Comp., 32, 59 (1996)].
S. V. Chapyshev, Mendeleev Commun., 9, 164 (1999).
S. V. Chapyshev and V. M. Anisimov, Khim. Geterotsikl. Soedin., 1521 (1997). [Chem. Heterocycl. Comp., 33, 1315 (1997)].
S. V. Chapyshev, Khim. Geterotsikl. Soedin., 1497 (2000). [Chem. Heterocycl. Comp., 36, 1289 (2000)].
S. V. Chapyshev, Khim. Geterotsikl. Soedin., 935 (2001). [Chem. Heterocycl. Comp., 37, 861 (2001)].
S. V. Chapyshev, R. Walton, and P. M. Lahti, Mendeleev Commun., 10, 187 (2000).
S. V. Chapyshev, Khim. Geterotsikl. Soedin., 87 (2003). [Chem. Heterocycl. Comp., 39, 83 (2003)].
S. V. Chapyshev and M. S. Platz, Mendeleev Commun., 11, 56 (2001).
S. V. Chapyshev, Mendeleev Commun., 9, 166 (1999).
E. Keßenich, T. M. Klapötke, J. Knizek, H. Nöth, and A. Schulz, Eur. J. Inorg. Chem., 2013 (1998).
D. R. Miller, D. C. Swenson, and E. G. Gillan, J. Am. Chem. Soc., 126, 5372 (2004).
C. Ye, H. Gao, J. A. Boatz, G. W. Drake, B. Twamley, and J. M. Shreeve, Angew. Chem. Int. Ed. Engl., 45, 7262 (2006).
S. I. Kuzina, D. V. Korchagin, G. V. Shilov, S. V. Chapyshev, A. I. Mikhailov, and S. M. Aldoshin, Doklady Phys. Chem., 418, 7 (2008).
S. I. Kuzina, D. V. Korchagin, G. V. Shilov, A. I. Mikhailov, S. V. Chapyshev, and S. M. Aldoshin, Russ. J. Phys. Chem. A, 82, 1870 (2008).
S. M. Aldoshin, D. V. Korchagin, K. V. Bozhenko, G. V. Shilov, and S. V. Chapyshev, Bull. Russ. Acad. Sci.: Physics, 72, 1556 (2008).
S. V. Chapyshev, V. F. Lavitskii, A. V. Akimov, E. Ya. Misochko, A. V. Shastin, D. V. Korchagin, G. V. Shilov, and S. M. Aldoshin, Russ. Chem. Bull., 57, 534 (2008).
R. H. Abu-Eittah and M. K. Khedr, J. Mol. Struct.: THEOCHEM, 822, 74 (2007).
A. M. Sipyagin and B. V. Kunchenko, Khim. Geterotsikl. Soedin., 657 (1994). [Chem. Heterocycl. Comp., 30, 576 (1994)].
A. M. Sipyagin and I. V. Efremov, Khim. Geterotsikl. Soedin., 1088 (1996). [Chem. Heterocycl. Comp., 32, 937 (1996)].
G. M. Sheldrick, SHELXTL v. 6.14, Structure Determination Software Suite, Bruker AXS, Madison, Wis., USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 7, pp. 998–1008, July, 2011.
Rights and permissions
About this article
Cite this article
Chapyshev, S.V., Korchagin, D.V., Shilov, G.V. et al. Synthesis and structure of asymmetric 2,4,6-triazidopyridines. Chem Heterocycl Comp 47, 817–825 (2011). https://doi.org/10.1007/s10593-011-0841-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-011-0841-x