Abstract
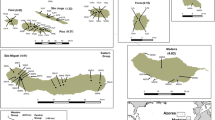
Translocation and reintroduction are important tools for the conservation or recovery of species threatened with extinction in the wild. However, an understanding of the potential genetic consequences of mixing populations requires an understanding of the genetic variation within, and similarities among, donor and recipient populations. Genetic diversity was measured using two independent marker systems (microsatellites and AFLPs) for one island and four small remnant mainland populations of Setonix brachyurus, a threatened medium sized macropod restricted to fragmented habitat remnants and two off-shore islands in southwest Australia. Microsatellite diversity in the island population (R s = 3.2, H e = 71%) was similar to, or greater than, all mainland populations (R s = 2.1–3.9, H e = 34-71%). In contrast, AFLP diversity was significantly lower in the island population (PPL = 20.5; H j = 0.118) compared to all mainland populations (mean PPL = 79.5–89.7; mean H j = 0.23–0.29). Microsatellites differentiated all (mainland and island) populations from each other. However, AFLP only differentiated the island population from the mainland populations—all mainland populations were not significantly differentiated from each other for this marker. Given a known time since isolation of the island population from the mainland (6,000 years ago), and an overall more conservative rate of evolution of AFLP markers, our results are consistent with mainland populations fragmenting thousands of years ago (but <6,000 years), probably as a consequence of reduced rainfall and the constriction of the preferred mesic habitat of quokkas. Our results also support a recent history of severe population bottlenecks in mainland populations, and a long history of bottlenecks of the island population, but reflect a recent explosion in numbers since European occupation of the island. Our results indicate that translocation of island populations to supplement mainland populations would introduce genetically markedly differentiated, and possibly maladapted, individuals.



Similar content being viewed by others
References
Abbott I (2008) Historical perspectives of the ecology of some conspicuous vertebrate species in south-west Western Australia. Conservation Science W Aust 6:1–214
Antao T, Lopes A, Lopes RJ, Beja-Pereira A, Luikart G (2008) LOSITAN: a workbench to detect molecular adaptation based on a Fst-outlier method. BMC Bioinformatics 9:323
Armstrong DP, Davidson RS, Dimond WJ, Perrott JK, Castro I, Ewen JG, Griffiths R, Taylor J (2002) Population dynamics of reintroduced forest birds on New Zealand islands. J Biogeogr 29:609–621
Bailey NW, Macías Garcia M, Ritchie MG (2007) Beyond the point of no return? A comparison of genetic diversity in captive and wild populations of two nearly extinct species of Goodeid fish reveals that one is inbred in the wild. Heredity 98:360–367
Balme J, Merrilees D, Porter JK (1978) Late Quaternary mammal remains, spanning about 30 000 years, from excavations in Devil’s Lair, Western Australia. J R Soc West Aust 61:33–65
Bartley D, Bagley M, Gall G, Bentley N (1992) Use of linkage disequilibrium data to estimate effective size of hatchery and natural fish populations. Conserv Biol 6:365–375
Beaumont MA (1999) Detecting population expansion and decline using microsatellites. Genetics 153:2013–2029
Beaumont MA, Nichols RA (1996) Evaluating loci for use in the genetic analysis of population structure. Porc R Soc Lond B 263:1619–1626
Bensch S, Akesson M (2005) Ten years of AFLP in ecology and evolution: why so few animals? Mol Ecol 14:2899–2914
Bradshaw SD (ed) (1983) Research on Rottnest Island. J R Soc West Aust 66: parts 1 and 2
Brinkmann B, Klintschar M, Neuber F, Hühne J, Rolfe B (1998) Mutation rate in human microsatellites: influence of structure and length of the tandem repeat. Am J Hum Genet 62:1408–1415
Brown AH (1970) The estimation of Wright’s fixation index from genotypic frequencies. Genetica 41:399–406
Campbell D, Duchesne P, Bernatchez L (2003) AFLP utility for population assignment studies: analytical investigation and empirical comparison with microsatellites. Mol Ecol 12:1979–1991
Christensen P, Burrows N (1994) Project desert dreaming: experimental reintroduction of mammals to the Gibson Desert, Western Australia. In: Serena M (ed) Reintroduction biology of Australian, New Zealand fauna. Surrey Beatty and Sons, Sydney, pp 199–207
Christensen PES, Kimber PC (1975) Effect of prescribed burning on the flora and fauna of south-western Australian forests. Proc Ecol Soc Aust 9:85–106
Churchill DM (1959) Late Quarternary eustatic changes in the Swan River District. J Soc West Aust 42:53–55
de Tores PJ, Hayward MW, Dillon MJ, Brazell R (2007) Review of the distribution, causes for the decline and recommendations for management of the quokka, Setonix brachyurus (Macropodidae: Marsupialia), an endemic macropod marsupial from south-west Western Australia. Conservation Science West Aust 6:13–73
Edmands S, Timmermann CC (2003) Modelling factors affecting the severity of outbreeding depression. Conserv Biol 17:883–892
El Mousadik A, Petit RJ (1996) High levels of genetic differentiation for allelic richness among populations of the argan tree [Argania spinosa (L.) Skeels] endemic to Morroco. Theor Appl Genet 92:832–839
Eldridge MDB, King JM, Loupis AK, Spencer PBS, Taylor AC, Pope LC, Hall GP (1999) Unprecedented low levels of genetic variation and inbreeding depression in an island population of the black-footed rock-wallaby. Conserv Biol 13:531–541
Eldridge MDB, Kinnear JE, McKenzie LM, Orell P, Spencer PBS (2004) Genetic diversity in remnant mainland and ‘pristine’ island populations of three endemic Australian macropodids (Marsupialia): Macropus eugenii, Lagorchestes hirsutus and Petrogale lateralis. Conserv Genet 5:325–338
Estoup A, Angers B (1998) Microsatellites and minisatellites for molecular ecology: theoretical and experimental considerations. In: Carvallo G (ed) Advances in molecular ecology. NATO Press, Amsterdam
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial restriction sites. Genetics 131:479–491
Excoffier L, Laval G, Schneider S (2005) Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol Bioinform Online 1:47–50
Ferguson RJ (1986) Rottnest Island: history and architecture. University of Western Australia Press, Perth
Frankham R (1998) Inbreeding and extinction: island populations. Conserv Biol 12:665–675
Frankham R (2005) Genetics and extinction. Biol Conserv 126:131–140
Garza JC, Williamson EG (2001) Detection of reduction in population size using data from microsatellite loci. Mol Ecol 10:305–318
Gaudeul M, Till-Bottraud I, Barjon F, Manel S (2004) Genetic diversity and differentiation in Eryngium alpinum L. (Apiaceae): comparison of AFLP and microsatellite markers. Heredity 92:508–518
Goudet J (1995) FSTAT (Version 2.1): a computer program to calculate F-statistics. J Hered 86:485–486
Goudet J (2001) FSTAT, a program to estimate and test gene diversities and fixation indices (version 2.9.3). http://www.unil.ch/izea/softwares/fstat.html. Accessed 26 Mar 2009
Gubitz T, Thorpe RS, Malhotra A (2000) Phylogeography and natural selection in the Tenerife gecko Tarentola delalandii: testing historical and adaptive hypotheses. Mol Ecol 9:1213–1221
Guo SW, Thompson EA (1992) A Monte-Carlo method for combined segregation and linkage analysis. Am J Hum Genet 51:1111–1126
Hayward MW, de Tores PJ, Dillon M, Fox BJ (2003) Local population structure of a naturally occurring metapopulation of the quokka (Setonix brachyurus Macropodidae: Marsupialia). Biol Conserv 110:343–355
Hayward MW, de Tores PJ, Banks PA (2005) Habitat use of the quokka, Setonix brachyurus, (Macropodidae: Marsupialia), in the northern jarrah forest of Australia. J Mammal 86:683–688
Hayward MW, de Tores PJ, Dillon MJ, Banks PB (2007) Predicting the occurrence of the quokka, Setonix brachyurus (Macropodidae: Marsupialia), in Western Australia’s northern jarrah forest. Wildl Res 34:194–199
Hilton-Taylor C (2000) 2000 IUCN Red List of threatened species. IUCN. Gland, Switzerland and Cambridge, UK
Huff DR, Peakall R, Smouse PE (1993) RAPD variation within and among populations of outcrossing buffalograss (Buchloë dactyloides (Nutt.) Engelm.). Theor Appl Genet 86:927–934
Iwata H, Imon K, Tsumura Y, Ohsawa R (2005) Genetic diversity among Japanese indigenous common buckwheat (Fagopyrum esculentum) cultivars as determined from amplified fragment length polymorphism and simple sequence repeat markers and quantitative agronomic traits. Genome 48:367–377
Johnson KA, Burbridge AA, McKenzie NL (1989) Australian Macropodoidea: status, causes of decline and future research and management. In: Grigg G, Jarman P, Hume I (eds) Kangaroos, wallabies, and rat-kangaroos, vol Vol.2. Surrey Beatty & Sons Pty Limited. Chipping Norton, New South Wales, pp 641–657
King DR, Smith LA (1985) The distribution of the European red fox (Vulpes vulpes) in Western Australia. Records of the Western Australian Museum 12:197–205
Krauss S (1999) Complete exclusion of non-sires in an analysis of paternity in a natural plant population using amplified fragment length polymorphism (AFLP). Mol Ecol 8:217–226
Lacy RC (1997) The importance of genetic variability to the viability of mammalian populations. J Mammal 78:320–335
Maguire TL, Peakall R, Saenger P (2002) Comparative analysis of genetic diversity in the mangrove species Avicenna marina (Forsk.) Vierh. (Avicenniacea) detected by AFLPs and SSRs. Theor Appl Genet 104:388–398
Main AR, Yadav M (1971) Conservation of macropods in reserves in Western Australia. Biol Conserv 3:123–133
Mariette S, Chagné D, Lézier C, Pastuszka P, Raffin A, Plomion C, Kremer A (2001) Genetic diversity within and among Pinus pinaster populations: comparison between AFLP and microsatellite markers. Heredity 86:469–479
Mariette S, Le Corre V, Austerlitz F, Kremer A (2002a) Sampling within the genome for measuring within-population diversity: trade-offs between markers. Mol Ecol 11:1145–1156
Mariette S, Cottrell J, Csaikl UM, Goikoecha P, König A, Lowe AJ, Van Dam BC, Barreneche T, Bodénès C, Streiff R, Burg K, Groppe K, Muro RC, Tabbener H, Kremer A (2002b) Comparison of levels of genetic diversity detected with AFLP and microsatellite markers within and among mixed Q. petraea (MATT.) LIEBL. And Q. robur L. stands. Silvae Genetica 51:72–79
Marshall TC, Spalton JA (2000) Simultaneous inbreeding and outbreeding depression in reintroduced Arabian oryx. Anim Conserv 3:241–248
Michalakis Y, Excoffier L (1996) A generic estimation of population subdivision using distances between alleles with special reference for microsatellite loci. Genetics 142:1061–1064
Miller MP (1997) Tools for population genetic analysis (TFPGA) 1.3: a Windows program for the analysis of allozyme and molecular population genetic data. North Arizona University, Flagstaff, AZ
Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16:1215
Mills HR, Moro D, Spencer PBS (2004) Conservation significance of island versus mainland populations: a case study of dibblers (Parantechinus apicalis) in Western Australia. Anim Conserv 7:387–395
Mock KE, Theimer TC, Rhodes OE Jr, Greenberg DL, Keim P (2002) Genetic variation across the historical range of the wild turkey (Meleagris gallopavo). Mol Ecol 11:643–657
Moritz C (1994) Defining “Evolutionary Significant Units’ for conservation. Trends Ecol Evol 9:373–375
Moritz C (1999) Conservation units and translocations: strategies for conserving evolutionary processes. Hereditas 130:217–228
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
Nybom H (2004) Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol Ecol 13:1143–1155
Orloci L (1978) Multivariate analysis in vegetation research. Dr W. Junk B. V, The Hague-Boston
Peakall R, Smouse PE (2001) GenAlEx V5: genetic analysis in Excel. Population genetic software for teaching and research. Australian National University, Canberra, Australia. http://www.anu.edu.au/BoZo/GenAlEx/. Accessed 26 Mar 2009
Peakall R, Smouse PE, Huff DR (1995) Evolutionary implications of allozyme and RAPD variation in diploid populations of buffalograss. Buchloë dactyloides (Nutt. Engelm.). Mol Ecol 4:135–147
Peel D, Ovenden JR, Peel SL (2004) NeEstimator: software for estimating effective population size. Molecular Fisheries Laboratory, Queensland Department of Primary Industries, Australia
Perry DH (1973) Some notes on the decline and subsequent recovery of mammal populations in the South-West. W Aust Nat 12:128–130
Pope LC, Sharp A, Moritz C (1996) Population structure of the yellow-footed rock-wallaby Petrogale xanthopus (Gray, 1854) inferred from mtDNA sequences and microsatellite loci. Mol Ecol 5:629–640
Pope LC, Estoup A, Moritz C (2000) Phylogeography and population structure of an ecotonal marsupial, Bettongia tropica, determined using mtDNA and microsatellites. Mol Ecol 9:2041–2053
Portis E, Barchi L, Acquadro A, Macua JI, Lanteri S (2005) Genetic diversity assessment in cultivated cardoon by AFLP (amplified fragment length polymorphism) and microsatellite markers. Plant Breed 124:199–304
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population genetic structure using multilocus genotypes. Genetics 155:945–959
Raymond M, Rousset F (1995) An exact test for population differentiation. Evolution 49:1280–1283
Richards C, Leberg PL (1995) Temporal changes in allele frequencies and a population’s history of severe bottlenecks. Conserv Biol 10:832–839
Riddle BR, Honerycut RL, Lee PL (1993) Mitochondrial DNA phylogeny in northern grasshopper mice (Onychomys leucogaster)–the influence of Quaternary climatic oscillations on population dispersion and divergence. Mol Ecol 2:183–193
Ryan PG, Siegfried WR (1994) The viabilitiy of small populations of birds: an empirical investigation of vulnerability. Forschungsberichte–Nationalpark Berchtesgaden 27:6–19
Schlotterer C, Pemberton JM (1994) The use of microsatellites for genetic analysis of natural populations. In: Schierwater B, Streit B, Wagner G, DeSalle R (eds) Molecular ecology and evolution: approaches and applications. Birkhauser Verlag, Basel/Switzerland, pp 203–214
Sinclair EA (1998) Morphological variation in the quokka, Setonix brachyurus (Marsupialia: Macropodidae). Aust J Zool 46:439–449
Sinclair EA (2001) Phylogeographic variation in the quokka, Setonix brachyurus (Marsupialia: Macropodidae): implications for conservation. Anim Conserv 4:325–333
Sinclair E, Morris K (1996) Where have all the quokkas gone? Landscope Summer: 49–53
Spencer PBS, Adams M, Marsh H, Miller DJ, Eldridge M (1997) High levels of genetic variability in an isolated colony of rock-wallabies (Petrogale assimilis): evidence from three classes of molecular markers. Aust J Zool 45:199–210
Spencer PBS, Cardosa M, How RA, Williams J, Bunce M, Schmitt LH (2007) Cross-species amplification at microsatellite loci in Australian quolls including the description of five new markers from the Chuditch (Dasyurus geoffroii). Mol Ecol Notes 7:1100–1103
Storr GM (1964) Studies on marsupial nutrition. 4. Diet of the quokka, Setonix brachyurus (Quoy and Gaimard) on Rottnest Island, Western Australia. Aust J Biol Sci 17:469–481
Storr GM (1965) Notes on Bald Island and the adjacent mainland. W Aust Nat 9:187–196
Tallmon DA, Luikart G, Waples RS (2004) The alluring simplicity and complex reality of genetic rescue. Trends Ecol Evol 19:489–496
Taylor AC, Cooper DW (1998) A set of unlinked microsatellites from the tammar wallaby, Macropus eugenii. Mol Ecol 7:925–926
Taylor AC, Sherwin WB, Wayne RK (1994) Genetic variation of microsatellite loci in the northern hairy-nosed wombat Lasiorhinus lorefftii. Mol Ecol 3:227–290
Vekemans X (2002) AFLP-SURV 1.0. A program for genetic diversity analysis with AFLP (and RAPD) population data. Université Libre de Bruxelles, Belgium
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
White SR (1952) The occurrence of the quokka in the south-west. W Aust Nat 3:101–103
Williamson-Natesan EG (2005) Comparison of methods for detecting bottlenecks from microsatellite loci. Conserv Genet 6:551–562
Yeh FC, Boyle TJB (1997) Population genetic analysis of co-dominant and dominant markers and quantitative traits. Belg J Bot 129:157
Zhang DX, Hewitt GM (2003) Nuclear DNA analyses in genetic studies of populations: practice, problems and prospects. Mol Ecol 12:563–584
Zhivotovsky LA (1999) Estimating population structure in diploids with multilocus dominant DNA markers. Mol Ecol 8:907–913
Acknowledgments
This project was funded by the Pest Animal Control Co-operative Research Centre. We thank Mike Calver, Liz Sinclair and the reviewers for suggestions that improved the manuscript and also gratefully acknowledge support from Matt Hayward, the Western Australian Department of Environment and Conservation (DEC), and the Marsupial CRC.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See also Table 7.
Rights and permissions
About this article
Cite this article
Alacs, E.A., Spencer, P.B.S., de Tores, P.J. et al. Population genetic structure of island and mainland populations of the quokka, Setonix brachyurus (Macropodidae): a comparison of AFLP and microsatellite markers. Conserv Genet 12, 297–309 (2011). https://doi.org/10.1007/s10592-010-0140-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-010-0140-6