Abstract
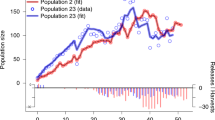
Maintaining genetic variation and minimizing inbreeding are central goals of conservation genetics. It is therefore crucial to understand the important population parameters that affect inbreeding, particularly in reintroduction programs. Using data from 41 reintroduced Alpine ibex (Capra ibex ibex) populations we estimated inbreeding since the beginning of reintroductions using population-specific Fst, and inbreeding over the last few generations with contemporary effective population sizes. Total levels of inbreeding since reintroduction of ibex were, on average, close to that from one generation of half-sib mating. Contemporary effective population sizes did not reflect total inbreeding since reintroduction, but 16% of variation in contemporary effective population sizes among populations was due to variation in current population sizes. Substantial variation in inbreeding levels among populations was explained by founder group sizes and the harmonic mean population sizes since founding. This study emphasizes that, in addition to founder group sizes, early population growth rates are important parameters determining inbreeding levels in reintroduced populations.






Similar content being viewed by others
References
Allendorf FW, Luikart G (2007) Conservation and the genetics of populations. Blackwell Publishing, Oxford
Allendorf FW, England PR, Luikart G, Ritchie PA, Ryman N (2008) Genetic effects of harvest on wild animal populations. Trends Ecol Evol 23:327–337
Anderson EC (2005) An efficient Monte Carlo method for estimating Ne from temporally spaced samples using a coalescent-based likelihood. Genetics 170:955–967
Ardren WR, Kapuscinski AR (2003) Demographic and genetic estimates of effective population size (Ne) reveals genetic compensation in steelhead trout. Mol Ecol 12:35–49
Aspi J, Roininen E, Ruokonen M, Kojola I, Vila C (2006) Genetic diversity, population structure, effective population size and demographic history of the Finnish wolf population. Mol Ecol 15:1561–1576
Bartley D, Bagley M, Gall G, Bentley B (1992) Use of linkage disequilibrium data to estimate effective size of hatchery and natural fish populations. Conserv Biol 6:365–375
Biebach I, Keller LF (2009) A strong genetic footprint of the reintroduction history of Alpine ibex (Capra ibex ibex). Mol Ecol 18:5046–5058
Chesser RK, Rhodes OE, Sugg DW, Schnabel A (1993) Effective sizes for subdivided populations. Genetics 135:1221–1232
Ciofi C, Beaumont MA, Swingland IR, Bruford MW (1999) Genetic divergence and units for conservation in the Komodo dragon Varanus komodoensis. Proc R Soc Lond B Biol Sci 266:2269–2274
Crow JF, Kimura M (1970) An introduction to population genetics theory. Burgess Publishing Company, Minneapolis, MN
Ewing SR, Nager RG, Nicoll MAC, Aumjaud A, Jones CG, Keller LF (2008) Inbreeding and loss of genetic variation in a reintroduced population of Mauritius Kestrel. Conserv Biol 22:395–404
Frankham R (1995) Effective population-size adult-population size ratios in wildlife––a review. Genet Res 66:95–107
Frankham R, Ballou JD, Briscoe DA (2002) Introduction to conservation genetics. Cambridge University Press, Cambridge
Fraser DJ, Hansen MM, Ostergaard S, Tessier N, Legault M, Bernatchez L (2007) Comparative estimation of effective population sizes and temporal gene flow in two contrasting population systems. Mol Ecol 16:3866–3889
Grodinsky C, Stuwe M (1987) The reintroduction of the Alpine Ibex to the Swiss Alps. Smithsonian 18:68–77
Hedrick P (2005) Large variance in reproductive success and the N-e/N ratio. Evolution 59:1596–1599
Hedrick PW, Miller PS (1992) Conservation genetics––techniques and fundamentals. Ecol Appl 2:30–46
Hill WG (1981) Estimation of effective population-size from data on linkage disequilibrium. Genet Res 38:209–216
Hoelzel AR (1999) Impact of population bottlenecks on genetic variation and the importance of life-history: a case study of the northern elephant seal. Biol J Linn Soc 68:23–39
Holsinger KE, Weir BS (2009) Genetics in geographically structured populations: defining, estimating and interpreting Fst. Nat Rev Genet 10:639–650
Hudson RR (1985) The sampling distribution of linkage disequilibrium under an infinite allele model without selection. Genetics 109:611–631
Jacquard A (ed) (1974) The genetic structure of populations. Springer Verlag, Berlin
Jacquard A (1975) Inbreeding: one word, several meanings. Theor Popul Biol 7:338–363
Johnson PCD, Haydon DT (2007) Maximum-likelihood estimation of allelic dropout and false allele error rates from microsatellite genotypes in the absence of reference data. Genetics 175:827–842
Keller LF, Waller DM (2002) Inbreeding effects in wild populations. Trends Ecol Evol 17:230–241
Keller LF, Biebach I, Hoeck PEA (2007) The need for a better understanding of inbreeding effects on population growth. Anim Conserv 10:286–287
Leberg P (2005) Genetic approaches for estimating the effective size of populations. J Wildl Manag 69:1385–1399
Maudet C, Beja-Pereira A, Zeyl E, Nagash H, Kence A, Ozut D, Biju-Duval MP, Boolormaa S, Coltman DW, Taberlet P, Luikart G (2004) A standard set of polymorphic microsatellites for threatened mountain ungulates (Caprini, Artiodactyla). Mol Ecol Notes 4:49–55
Mitchell RJ (1993) Path analysis––pollination. In: Scheiner SM, Gurevitch J (eds) The design and analysis of ecological experiments, 1st edn. Chapman & Hall, New York, pp 211–230
Nei M, Li WH (1973) Linkage disequilibrium in subdivided populations. Genetics 75:213–219
Nei M, Tajima F (1981) Genetic drift and estimation of effective population size. Genetics 98:625–640
Nei M, Maruyama T, Chakraborty R (1975) Bottleneck effect and genetic-variability in populations. Evolution 29:1–10
Nunney L (1991) The influence of age structure and fecundity on effective population-size. Proc R Soc Lond B Biol Sci 246:71–76
Nunney L (1993) The influence of mating system and overlapping generations on effective population-size. Evolution 47:1329–1341
Palstra FP, Ruzzante DE (2008) Genetic estimates of contemporary effective population size: what can they tell us about the importance of genetic stochasticity for wild population persistence? Mol Ecol 17:3428–3447
R Development Core Team (2006) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ryman N, Baccus R, Reuterwall C, Smith MH (1981) Effective population-size, generation interval, and potential loss of genetic-variability in game species under different hunting regimes. Oikos 36:257–266
Saether BE, Lillegard M, Grotan V, Filli F, Engen S (2007) Predicting fluctuations of reintroduced ibex populations: the importance of density dependence, environmental stochasticity and uncertain population estimates. J Anim Ecol 76:326–336
Schaub M, Zink R, Beissmann H, Sarrazin F, Arlettaz R (2009) When to end releases in reintroduction programmes: demographic rates and population viability analysis of bearded vultures in the Alps. J Appl Ecol 46:92–100
Stiver JR, Apa AD, Remington TE, Gibson RM (2008) Polygyny and female breeding failure reduce effective population size in the lekking Gunnison sage-grouse. Biol Conserv 141:472–481
Stuwe M, Scribner KT (1989) Low genetic-variability in reintroduced Alpine Ibex (Capra ibex ibex) populations. J Mammal 70:370–373
Vitalis R, Couvet D (2001) Estimation of effective population size and migration rate from one- and two-locus identity measures. Genetics 157:911–925
Vitalis R, Dawson K, Boursot P (2001) Interpretation of variation across marker loci as evidence of selection. Genetics 158:1811–1823
Wang JL (2001) A pseudo-likelihood method for estimating effective population size from temporally spaced samples. Genet Res 78:243–257
Wang JL (2005) Estimation of effective population sizes from data on genetic markers. Philos Trans R Soc B Biol Sci 360:1395–1409
Waples RS (1989) A generalized approach for estimating effective population size from temporal changes in allele frequency. Genetics 121:379–391
Waples RS (2002) Definition and estimation of effective population size in the conservation of endangered species. In: Beissinger SR, McCullough DR (eds) Population viability analysis. University of Chicago Press, Chicago, pp 147–168
Waples RS (2005) Genetic estimates of contemporary effective population size: to what time periods do the estimates apply? Mol Ecol 14:3335–3352
Waples RS, Do C (2008) LDNE: a program for estimating effective population size from data on linkage disequilibrium. Mol Ecol Resour 8:753–756
Weir BS, Cockerham CC (1984) Estimating f-statistics for the analysis of population-structure. Evolution 38:1358–1370
Wright S (1969) Evolution and the genetics of populations, Vol 2. The theory of gene frequencies. University of Chicago Press, Chicago
Acknowledgements
This study would not have been possible without the help of numerous game wardens who collected samples and assisted with biopsy darting. We thank the hunting agencies of the cantons Appenzell Innerrhoden, Bern, Glarus, Graubünden, Luzern, Nidwalden, Obwalden, Schwyz, St. Gallen, Tessin, Uri, Vaud and Valais for their support in collecting data and samples. Kim Scribner kindly supplied samples from 1986 to 1988 and patiently answered our questions about them. Thanks to Mark Beaumont for modifying 2MOD and for discussing inbreeding and Fst, to Heinz Maag for developing and producing biopsy darts, to Thomas Bucher for assistance with genotyping, to Peter Wandeler for advice on laboratory procedures, and to Simon Aeschbacher and Barabara Oberholzer for help gathering information about the reintroduction history. We thank Christine Grossen and Frank Reinisch for assistance in the field. This project was funded by the Swiss Federal Office for the Environment (FOEN) and the Forschungskredit of the University of Zurich and profited from valuable support from the ESF Science Networking Programme “ConGen”.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Table 2.
Rights and permissions
About this article
Cite this article
Biebach, I., Keller, L.F. Inbreeding in reintroduced populations: the effects of early reintroduction history and contemporary processes. Conserv Genet 11, 527–538 (2010). https://doi.org/10.1007/s10592-009-0019-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-009-0019-6