Abstract
The concept of liquid biopsy analysis has been established more than a decade ago. Since the establishment of the term, tremendous advances have been achieved and plenty of methods as well as analytes have been investigated in basic research as well in clinical trials. Liquid biopsy refers to a body fluid-based biopsy that is minimal-invasive, and most importantly, allows dense monitoring of tumor responses by sequential blood sampling. Blood is the most important analyte for liquid biopsy analyses, providing an easily accessible source for a plethora of cells, cell-derived products, free nucleic acids, proteins as well as vesicles. More than 12,000 publications are listed in PubMed as of today including the term liquid biopsy. In this manuscript, we critically review the current implications of liquid biopsy, with special focus on circulating tumor cells, and describe the hurdles that need to be addressed before liquid biopsy can be implemented in clinical standard of care guidelines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite tremendous efforts in clinical and basic cancer research, metastasis remains a key driver of cancer-associated mortality [1]. Therefore, early detection of both localized as well as metastasized cancers is of utmost importance for the survival of these patients. During metastasis, cancer cells detach from the primary tumor, infiltrate the local tissue, intravasate into blood vessels and ultimately invade into distant organs, where they need to be able to proliferate to form new metastases [2]. To achieve this, the tumor cells need to switch from an immotile to a more migratory state, a process called epithelial–mesenchymal transition (EMT), during which epithelial molecules are downregulated while simultaneously mesenchymal proteins are upregulated [3]. Moreover, EMT leads to the activation of several transcription factors such as Slug, Snail, Twist, and ZEB1/2 [3], ultimately resulting in alterations in signalling pathways changing polarity, cell-cell contacts and extracellular matrix composition, therefore enhancing invasive properties [4]. Once the detached cells arrive at the metastatic site, these traits need to be reversed through MET (mesenchymal to epithelial transition) to facilitate re-attachment, proliferation, and metastatic outgrowth [4].
Tumor cells that have undergone this process are called disseminated tumor cells (DTCs) and are frequently found in the bone marrow or lymph nodes. Other major, clinically relevant sites of metastases include the liver, the lung, the bone and the brain but are dependent on the anatomically determined lymphatic and venous drainage, the molecular characteristics of the primary tumor as well as the corresponding microenvironments of the secondary organs [5]. The presence of DTCs in the bone marrow negatively correlates with the survival of patients with various cancer entities [6, 7]. DTCs are tumor cells that have left the lymphatic or blood vessels and subsequently settled at lymph nodes or distant sites (e.g., bone marrow). DTCs occur at very low concentrations (e.g., 1 per 1 million bone marrow cells and highly sensitive methods are required for their detection (e.g., immunocytochemistry with keratin antibodies). Considering the total mass of bone marrow, it can be estimated that an early stage breast cancer patient at initial diagnosis can harbor hundred thousands of DTCs in the bone marrow and approximately 50% of these patients can control this tumor load over 10 years [8]. Understanding the mechanisms of this remarkable control might open new avenues for therapeutic interception aimed to block the development into full blown and incurable metastasis. To date, we know that DTCs can adapt to different microenvironments, reside in a non-proliferate (Ki67-negative) state, express oncogenes such as HER2, develop resistance against systemic anti-cancer treatment as well as acquire cancer stem cell (CSC)-like traits [9,10,11]. Bone marrow aspiration is an invasive procedure routinely used in patients with haematological malignancies but not in patients with solid tumors. Despite the promising clinical research studies on DTCs, the emerging interest in blood analyses (e.g., CTCs and ctDNA) has lowered the interest to implement DTC analyses into clinical protocols. However, we are confident that DTC analyses would add an important information that might be not obtained by minimally invasive blood tests which provide only a snap shot. Tumor cells that can be detected in the circulation are called circulating tumor cells (CTCs), however, as opposed to DTCs, they have not settled yet in a distant organ. For survival, these cells that have detached from the primary tumor or secondary metastatic sites circulate in the blood stream and have to withstand extreme conditions including high pressure within the vessels as well as evade immune surveillance mechanisms and adapt to a new microenvironment [12]. Unlike tissue biopsies, sampling of CTCs not only allows a comprehensive assessment of the state of the disease but also represents a non-invasive tool for sequential and real-time monitoring of tumor burden in cancer patients. Blood as a liquid biopsy source provides a convenient, easily accessible, and fast way of sampling and enables the investigation of different analytes apart from CTCs such as circulating nucleic acids (ctDNA, miRNAs), extracellular vesicles (EVs) and platelets. All analytes can provide complementary information on the (epi-)genomic, transcriptomic, and proteomic level which are highly valuable for the molecular characterization of the tumor burden in an individual cancer patient [13].
Analysis of CTCs to understand the biology of metastasis
While in early-stage cancer the primary tumor serves as the main source for CTCs, ctDNA and EVs (and in later stages also the lymph nodes), in more advanced cancers, metastases are mainly responsible for releasing CTCs and other cancer cell derived products into the circulation [14]. Using CTCs as liquid biomarkers presents a challenge, not only because of their short half-life of approximately 1-2.4 h [15] but also because especially in early stages the number of CTCs shed into the peripheral blood circulation is low compared to the vast background of blood cells. It is estimated that one CTC can be found per approximately 106-108 leukocytes [16], however, this number also strongly varies between the different tumor entities and disease stages. For analysis of CTCs, enrichment is thus indispensable and can be performed using a variety of devices and techniques.
By making use of the physical and mechanical properties of CTCs and leukocytes, separation of these two cell populations can for example be achieved through differences in size, deformability, density, or electric charges using devices, with new technologies being constantly developed [17].
Biological properties such as expression of specific cell surface markers on the other hand, are furthermore necessary to ultimately distinguish between CTCs and leukocytes and thus for their identification and enumeration and therefore prognostic and diagnostic value.
The most prominent CTC marker EpCAM (epithelial cell adhesion molecule) is currently being utilized in the only FDA-approved CTC detection platform to date, the CellSearch system, in which CTCs are enriched through adhesion to anti-EpCAM-conjugated magnetic beads. However, during metastasis, cells detaching from the tumor sites and undergoing EMT, often show a significant downregulation of epithelial proteins such as EpCAM [18]. Negative depletion of immune cells including anti-CD45 conjugates for leucocyte depletion is therefore another option of CTC enrichment. The epithelial ImmunoSPOT (EPISPOT) assays can be used to detect cancer-specific proteins secreted by viable CTCs such as cytokeratins, having proven to show prognostic value in breast, colon, prostate, and head and neck tumors and melanoma [17, 19]. Moreover, the EPIDROP assay is currently being developed for detection of CTCs in microdroplets at the single cell level [20].
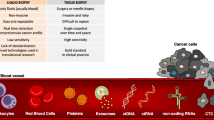
Through these various techniques, an enrichment of CTCs by several log scales can be achieved which is necessary for effective subsequent molecular characterization on a single cell level [20] (Fig. 1).
Overview of analytes in blood-based liquid biopsy analysis and CTC detection. Body fluids represent a rich source of cells as well as acellular products that can be assessed by liquid biopsy. Although liquid biopsy can be applied to all body fluids, the blood is most popular fluid within the field. Blood is composed of a cellular as well as acellular fraction. Within the soluble fraction, several liquid biopsy analytes including cell-free RNA, cell-free DNA, extracellular vesicles, (micro-)proteins as well as metabolites can be isolated. Circulating tumor cells (CTCs), if present, are surrounded by a plethora of other cells within the cellular fraction [14]. In order to isolate the CTCs from the blood sample, enrichment is required due to their low abundance. Several methods for CTC enrichment have been described in the past utilizing either biological properties of CTCs (in positive enrichment) such as marker expression or surrounding cells (depletion / negative enrichment) including immune cells. In addition to marker-based strategies, label-free methods based on physical properties have been established that allow the enrichment regardless of their marker expression for unbiased CTC detection. The enriched CTC fraction can be used for CTC enumeration but also for CTC molecular characterization as well as CTC cultivation for further insights into CTC biology [17, 19, 21]
For identification of CTCs, immunocytochemistry is often used to detect population-specific (e.g. EpCAM, cytokeratins, CD45), tissue- or tumor-specific or EMT-related antigen expression [17]. For a more extensive characterization, however, down-stream methods which allow for RNA or DNA analysis through e.g. RT-qPCR, RNAseq, whole genome amplification (WGA) and subsequent next generation sequencing (NGS) and/or proteomic analyses are needed to fully comprehend the state of the tumor. The detection of CTCs represents a highly specific blood-based technique that could be utilized for early detection strategies. However, as the number of CTCs is very low and enrichment procedures (negative, as well as positive enrichment) potentially lead to a loss of CTCs, the use of CTCs for early detection may lack sensitivity and negative predictive value. While positive enrichment methods based on cancer entities or primary tumor characteristics may provide a strong enrichment of CTCs, studies have also demonstrated a high degree of heterogeneity and discordance in primary tumor characteristics and detected CTCs. To increase the yield of CTCs, the use of larger blood volumes or diagnostic leukapheresis products (DLA) has been suggested. In metastatic breast cancer, an increase of almost 21-fold was observed by the authors comparing the CTC counts in peripheral blood with DLA products using the CellSearch platform. Strikingly, in two patients that had no detectable CTCs in the peripheral blood, CTCs were detected in the DLA products underlining the increase in sensitivity [22].
To further explore the functional properties of CTCs, suitable experimental models are needed. One good example for this is the establishment of in vitro CTC cell lines from the blood, even though being quite challenging. These model systems can be used to help understand the biology of CTCs, such as their heterogeneity in morphology, EMT status and features relevant for the development of metastasis [23]. Furthermore, more patient-relevant knowledge can be obtained, such as the (druggable) mutational status of both primary tumor and metastasis, evolutionary events, as well as potential drug sensitivities and resistances [23, 24].
In most studies, the average blood volume needed for CTC cell line establishment varies between 6 and 18 mL. Even though higher CTC counts from the same patient tend to have a higher success rate, which is often only the case in advanced stages, this property alone does not guarantee a successful establishment [23, 24]. For example, during the establishment of the first long-term stable CRC cell line, out of 71 patients only 2 patients (with 302 and 516 CTCs in their sample) gave rise to an CTC line ex vivo, of which one was only stable two months, whereas the latter was successfully established [25]. Nevertheless, Brungs et al. reported the establishment of a CTC cell line (UWG01CTC) from a distal oesophageal high grade neuroendocrine carcinoma patient with only 3 detectable CTCs (per 7.5 mL blood) [26]. In general, the success rates are relatively low, requiring several patient samples before successful establishment of a long-term stable CTC cell line [23, 27]. However, once established, CTC cell lines can serve as an excellent tool for drug screening applications and thus have the potential to become highly valuable in personalized medicine [27]. The use of DLA products can increase the yield and thereby the success rate of establishing an ex vivo CTC culture as described earlier by Mout et al. that reached a success rate of 35% for organoid expansion [28].
In contrast to commercially available, immortalized cell lines established from primary tumor tissue and metastatic sites, only a few CTC cell lines are available. In 2021, more than 300 successfully established CTC cell cultures have been reported, however, only less than 50 of these were stable for more than 6 months in culture [27]. Long-term stable CTC lines and also short-term cultivation of patient CTCs allow the analysis of viable cells and thereby pave the way towards a better understanding the biology of CTCs and identification of druggable targets.
The first permanent cell line derived from colon cancer CTCs (CTC-MCC-41) was successfully established by Cayrefourcq et al., which proved to harbor similar characteristics as the primary tumor [25]. The cell line was found to be highly sensitive towards AKT and mTOR inhibition, which suggests a possible benefit for the patient using targeted therapy approaches [29]. Moreover, in 2020, Koch et al. established a CTC cell line (CTC-ITB-01) from an ER+ breast cancer patient which showed high concordance between their primary CTCs. Furthermore, its CNA (copy number alteration) profile remains stable during culture. Of note, drug sensitivity testing of CTC-ITB-01 cells, also revealed a high susceptibility for CDK4/6 inhibitors, that was not administered to the patient who gave rise to the cell line and may represented a value treatment option [30]. Another noteworthy example of a long-term CTC cell line (> 2 years in culture) is the CTC-TJH-01 line, a non-small cell lung cancer CTC line, established by Que et al. [31]. The cell line exhibited an intermediate epithelial-mesenchymal phenotype, stem cell-like traits as well as an increased immune escape compared to established lung cancer cell lines including A549 [31]. Functionally, the authors demonstrated the tumorigenic and metastatic capacity in a murine xenotransplantation model in vivo after subcutaneous as well as intravenous (tail vein) injection. In line with the reported concordance of the primary tumor and CTCs in the CTC-ITB-01 cell line, the CTC-TJH-01 cell line shared wild type EGFR and a missense mutation at codon 12 of KRAS with the primary tumor [31].
These models thus prove to have a high prognostic value; however, their establishment remains challenging and is therefore only feasible in few selected patients. Using in vivo models, patient-derived xenografts (PDX) are furthermore suitable for the investigation of the potential to form metastases through injection of CTCs into immunodeficient mice. After intraductal injection of the CTC-ITB-01 cell line established by Koch et al., primary tumor growth was observed as well as spontaneous bone metastases, mirroring clinically relevant metastatic sites in ER+ breast cancer [30]. Consistently, Yu et al. reported the establishment of breast cancer CTC lines that retained ER positivity in 5 out of 6 lines. Moreover, in a murine subcutaneous xenotransplantation three CTC lines demonstrated tumorgenicity within 3 months with a matching molecular profile with regard to the respective primary tumor [32]. Similarly, the CTC-MCC-41 cell line as well as the corresponding primary tumor and xenografts harbor the same KRAS and BRAF mutations as well as CK20 expression [25]. The high capacity to retain the original molecular traits has also been confirmed in lung cancer, as demonstrated by CTC derived explants that were harvested after injection of CTCs into immunodeficient mice [33]. Faugeroux et al. established and characterized a prostate CTC-derived eXplant (CDX) model of model of neuroendocrine transdifferentiation in human castration resistant prostate cancer that allowed to study genetic and epigenetic mechanisms to further understand the biology of transdifferentiation and identify novel drug targets [34]. Overall, PDX models have shown metastasis formation in relevant organs and matching patient history which can be predictors of tumor development, ultimately representing a valuable tool in personalized medicine.
Circulating tumor cells in the clinical setting
The prognostic value of CTC detection has been extensively characterized in the past in different tumor entities. Detectable CTCs at the time of diagnosis are associated with reduced progression free and overall survival in many solid cancer entities [35,36,37]. In particular in breast cancer, there are numerous large-scale studies demonstrating a prognostic role of CTCs [38,39,40,41]. Similarly, for advanced/metastatic cancers or in relapse a negative prognosis has been already established in the past [42]. Although CTCs play a strong prognostic role in late stage cancers, the perspective of the use of CTC detection for early detection of cancer has been recently highlighted [43].
Molecular characterization of CTCs can also provide information on therapeutic targets. One example are the DETECT-III trials, the first randomized interventional study indicating clinical utility of CTC characterization that revealed that breast cancer patients with HER2 negative tumors have HER2 positive CTCs in the circulation. Strikingly, it has been demonstrated that these patients benefit from anti-HER2/anti-EGFR treatment using Lapatinib with regard to increased overall survival rates [44,45,46,47]. Although the reported CTC positivity rate of nearly two third [44] is consistent with other studies in metastatic breast cancer due to the EpCAM-based enrichment of the CellSearch system, EpCAMlow or EpCAM negative CTCs may be lost [48]. False-negative results for CTC positivity may be therefore reduced in the future by using additional CTC markers, label-free approaches, DLA products or in vivo capture systems [14, 49].
Despite the discordance of HER2 negative primary tumors and HER2 positive CTC reported in breast cancer, in lung cancer, a recent study analyzed the PD-L1 expression on CTCs, cytology imprints and tissue samples of NSCLC patients. The authors concluded that in clinical situations in which no tissue is available, the combination of cytological imprints and PD-L1 positive CTCs in the blood provides a predictive tool for tumoral PD-L1 status in NSCLC patients [50]. Moreover, a study on ALCAM expression in NSCLC patients with brain metastasis demonstrated that despite the high degree of heterogeneity of ALCAM expression on CTCs, a high concordance between ALCAM expression on CTCs and matched brain metastasis of the same patient was present [51].
For treatment monitoring, a meta-analysis also reported that in breast cancer patients CTC counts decrease after anti-tumor therapy and can therefore be used as an indicator of treatment response [52]. This may implicate the use of CTCs as a prognostic marker in the clinical routine or even implicate the ability to make use of the CTC counts as a real time marker for monitoring response rate while undergoing anti-tumor therapy.
Combination of liquid biopsy analytes
Due to the complementary nature of the different analytes found in the blood, numerous studies have demonstrated the great potential of combining CTC- and further liquid biomarker analyses for diagnostic and prognostic purpose [53]. However, the information obtained in these multiparameter studies greatly depends on the type of analyses being performed. Most studies use enumeration of CTCs which has been a marker for progression- and disease-free survival in singular studies of various cancer entities for over a decade and combined it with ctDNA detection which proved to enhance sensitivity and specificity [54,55,56,57].
On the other hand, analysis of genomic CTC DNA as a complementary analyte can give rise to a deeper understanding of the mutational state of the tumor cell and can thus provide insight into its spatial and temporal heterogeneity. Combined with ctDNA analysis, mutational profiling of both analytes was previously performed in several studies [58,59,60] and was found to reveal the same mutations in breast cancer patients [61, 62]. However, also mutations exclusively found in either one of the analytes were identified [61, 62]. Therefore, analysis of both sample types complements each other so that additional information can be obtained. Due to the diverse nature of analytes in the blood, the possibility of combining liquid analytes in not limited and thus, combined analysis of CTC with EVs (including their cargo), cell- free RNAs, circulating proteins, DNA methylation, platelets, as well as other cell types found in the circulation are all accounted in ongoing research and can add complimentary value [12, 63, 64].
A recent example of the combination of CTCs and tumor-derived EVs (tdEVs) has been published by Nanou et al. The authors examined the presence of tdEVs in both arms of the SWOG0500 trial (CTC response and non-response after the first treatment cycle). Nanou et al. demonstrated that the enumeration of tdEVs provides a strong prognostic marker complementary to the CTC response to further stratify patients into different risk groups [65].
Moreover, the feasibility of detecting more than two liquid analytes has already been successfully proven, however, pre-analytical parameters such as the choice of blood collection tube, and time to processing, among other factors, should be carefully taken into consideration [63, 66]. The combination of CTCs, ctDNA, EVs, proteins and metabolites is currently being assessed in the EU-funded PANCAID consortium [67] with the goal to detect pancreatic adenocarcinomas earlier than current imaging tools.
Standardization of liquid biopsy analysis and current clinical recommendations
Since the discovery of liquid biopsy as a tool for cancer diagnostics and prognostics over a decade ago [68], a multitude of isolation and analysis techniques have been developed. Furthermore, novel biomarkers such as CAFs, or CECs that can be found in the blood circulation are the topic of ongoing investigations and help to characterize the tumor (and its tumor microenvironment) more precisely, providing a deeper understanding on the state of the disease, and possible treatment options.
However, these advances also add a new level of complexity to clinical decision making. For a successful implementation into clinical practice, numerous pre-analytical and analytical factors must be taken into consideration [69]. Parameters such as sampling method and consumables, storage time, storage temperature, and moreover patient medication, time of sampling and comorbidities can all have an influence on the liquid analytes [66, 70]. Furthermore, a deep understanding of the correct choice of analyte and assay is urgently required to obtain and interpret the information suited for the respective patient and cancer entity. This multitude of parameters contributes to the lack of standardization and represents the biggest hurdle on the way to clinical implementation, when in fact, clinical practice needs guidelines and needs to be reliable and comparable.
To this day, numerous CTC detection methods are available and are used in research studies. Several efforts have been made to evaluate these methods to assess their technical validity and enhance their applicability in a clinical setting [71, 72]. Koch et al. demonstrated the necessity of studying pre-analytical and analytical parameters, and furthermore defining optimal conditions for CTC enrichment [70]. In a study by Lampignano et al. initiated by the CANCER-ID/ELBS consortium [73] different technologies for ctDNA purification, quantification, and characterization were evaluated in a multi-center study. Using spike-in experiments of TP53 mutated lung cancer cell line DNA in healthy donor blood, the study demonstrated the feasibility of mutation detection by NGS and ddPCR independent of the laboratory [74].
Despite these challenges, several liquid biopsy tests have already found their way into clinical practice.
The presence of CTCs has been shown to be a negative prognostic marker for overall survival and can be used to identify those patients at risk for relapse or progression [75]. Their clinical importance of CTC detection was acknowledged by the American Joint Committee’s (AJCC). Their breast cancer staging manual was updated in 2018 and includes the a new classification, cM0(i+), which is used to describe the presence of CTCs and or DTC clusters (≤ 0.2 mm) in the bone marrow or other organs distant from regional lymph nodes and the breast [76].
Moreover, the detection and genomic analysis of ctDNA has been proven to be a valuable tool in personalized medicine [77], especially with the recent advances in targeted therapy as well as immune checkpoint inhibition (ICI). The European Society for Medical Oncology (ESMO) therefore issued recommendations on the usage of ctDNA based assays for genotyping and treatment selection due to their high validity and clinical utility [78]. The detection of targetable mutations, e.g. EGFR in NSCLC or KRAS/NRAS/BRAF V600E in colorectal cancers, could lead to a faster diagnosis and treatment selection and could be taken into account when tissue biopsies are not available. Taken together, these recent advances have led to the implementation of several tests into the clinical routine including the analysis of targetable EGFR mutations and ALK rearrangements in NSCLC ctDNA [79], analysis of AR-V7 status in CTCs from mCRPC patients [80], and also multigene assays such as the NGS-based Guardant360 CDx [81]. Recent studies have also shown clinical utility of CTC enumeration and characterization for therapeutic decision making in metastatic breast cancer [82, 83].
However, the possibility of false-positive findings needs to be considered which might derive from clonal expansion of apoptotic hematopoietic cells in the blood (clonal hematopoiesis of indeterminate potential (CHIP)) [84]. False-negative results could furthermore occur when only low levels of plasma DNA are available, or the tumor does not shed sufficient DNA [78]. To address these challenges for the detection of MRD in colorectal, pancreatic and lung cancer the EU/IHI-funded GUIDE.MRD consortium was initiated in 2023 [85, 86].
Conclusions
In conclusion, there are still hurdles that need to be addressed before liquid biopsy tests will find their way into routine clinical practice. High costs for detection devices and personnel as well as their training, and the constant need for tumor surveillance due to its spatial and temporal heterogeneity are examples for the challenges that are faced today.
To overcome these obstacles, several international projects such as CANCER-ID (2015–2019), which was carried out under the umbrella of the European Union Innovative Medicines Initiative (IMI), have been established to standardize protocols for clinical validation of liquid biopsy methods. Apart from that, EU-wide as well as internationally active consortia and networks such as the European Liquid Biopsy Society (ELBS) provide a network of academic and industry partners which aim at closing the gap between the multitude of scientific publications and clinical implementation of liquid biopsy [87]. Moreover, international efforts are currently being made to update the RECIST criteria (Response Evaluation Criteria In Solid Tumors) by including ctDNA analysis for treatment response evaluation in solid tumors [88].
Despite the many challenges that still need to be overcome, the current advances in research and the joint efforts of academia and industry partners pave the way for the establishment of a successful clinical implementation of liquid biopsy. These endeavours will ultimately change cancer treatment through improvements in early detection, risk assessment and patient monitoring to prolong survival rates and enhance quality of life of many patients in the future.
Abbreviations
- AJCC:
-
American Joint Committee
- CAF:
-
Cancer-associated fibroblast
- CDX:
-
CTC-derived eXplant
- CEC:
-
Circulating endothelial cell
- CHIP:
-
Clonal hematopoiesis of indeterminate potential
- CNA:
-
Copy number alteration
- CSC:
-
Cancer stem cell
- CTC:
-
Circulating tumor cell
- DTC:
-
Disseminated tumor cell
- ELBS:
-
European Liquid Biopsy Society
- EMT:
-
Epithelial–mesenchymal transition
- EpCAM:
-
Epithelial cell adhesion molecule
- EPISPOT:
-
Epithelial ImmunoSPOT
- ER:
-
Estrogen receptor
- ESMO:
-
European Society for Medical Oncology
- EV:
-
Extracellular vesicle
- FDA:
-
Food and Drug Administration
- ICI:
-
Immune checkpoint inhibition
- ILSA:
-
International Liquid Biopsy Standardization Alliance
- IMI:
-
Innovative Medicines Initiative
- NGS:
-
Next generation sequencing
- NSCLC:
-
Non-small cell lung cancer
- MET:
-
Mesenchymal-epithelial transition
- PDX:
-
Patient-derived xenograft
- RECIST:
-
Response Evaluation Criteria In Solid Tumors
- tdEVs:
-
Tumor-derived EVs
- WGA:
-
Whole genome amplification
References
Fares J et al (2020) Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther 5(1):28
Massagué J, Obenauf AC (2016) Metastatic colonization by circulating tumour cells. Nature 529(7586):298–306
Thiery JP et al (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139(5):871–890
Nieto MA et al (2016) EMT: 2016. Cell 166(1):21–45
Obenauf AC, Massagué J (2015) Surviving at a Distance: Organ-Specific Metastasis. Trends Cancer 1(1):76–91
Werner S, Heidrich I, Pantel K (2022) Clinical management and biology of tumor dormancy in breast cancer. Semin Cancer Biol 78:49–62
Hofbauer LC et al (2021) Novel approaches to target the microenvironment of bone metastasis. Nat Rev Clin Oncol 18(8):488–505
Braun S et al (2005) A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 353(8):793–802
Hen O, Barkan D (2020) Dormant disseminated tumor cells and cancer stem/progenitor-like cells: similarities and opportunities. Sem Cancer Biol 60:157–165
Dasgupta A, Lim AR, Ghajar CM (2017) Circulating and disseminated tumor cells: harbingers or initiators of metastasis? Mol Oncol 11(1):40–61
Pantel K et al (1993) Differential expression of proliferation-associated molecules in individual micrometastatic carcinoma cells. J Natl Cancer Inst 85(17):1419–1424
Lianidou E, Pantel K (2019) Liquid biopsies. Genes Chromosomes Cancer 58(4):219–232
Joosse SA, Pantel K (2015) Tumor-educated platelets as Liquid Biopsy in Cancer patients. Cancer Cell 28(5):552–554
Keller L, Pantel K (2019) Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat Rev Cancer 19(10):553–567
Meng S et al (2004) Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res 10(24):8152–8162
Barradas AM, Terstappen LW (2013) Towards the Biological understanding of CTC: capture technologies, definitions and potential to create metastasis. Cancers (Basel) 5(4):1619–1642
Alix-Panabieres C, Pantel K (2021) Liquid Biopsy: from Discovery to Clinical Application. Cancer Discov 11(4):858–873
Hyun KA et al (2016) Epithelial-to-mesenchymal transition leads to loss of EpCAM and different physical properties in circulating tumor cells from metastatic breast cancer. Oncotarget 7(17):24677–24687
Alix-Panabieres C (2012) EPISPOT assay: detection of viable DTCs/CTCs in solid tumor patients. Recent Results Cancer Res 195:69–76
Pantel K, Alix-Panabieres C (2019) Liquid biopsy and minimal residual disease - latest advances and implications for cure. Nat Rev Clin Oncol 16(7):409–424
Eslami-S Z et al (2022) Functional analysis of circulating tumour cells: the KEY to understand the biology of the metastatic cascade. Br J Cancer 127(5):800–810
Franken A et al (2019) Label-Free Enrichment and Molecular characterization of viable circulating tumor cells from Diagnostic Leukapheresis products. Clin Chem 65(4):549–558
Shimada Y et al (2022) Cell lines of circulating Tumor cells: what is known and what needs to be resolved. J Personalized Med 12(5):666
Tayoun T et al (2019) CTC-derived models: a window into the seeding capacity of circulating tumor cells (CTCs). Cells 8(10):1145
Cayrefourcq L et al (2015) Establishment and characterization of a cell line from human circulating colon cancer cells. Cancer Res 75(5):892–901
Brungs D et al (2020) Establishment of novel long-term cultures from EpCAM positive and negative circulating tumour cells from patients with metastatic gastroesophageal cancer. Sci Rep 10(1):539
Smit DJ, Pantel K, Jücker M (2021) Circulating tumor cells as a promising target for individualized drug susceptibility tests in cancer therapy. Biochem Pharmacol 188:114589
Mout L et al (2021) Generating human prostate cancer organoids from leukapheresis enriched circulating tumour cells. Eur J Cancer 150:179–189
Smit DJ et al (2020) High sensitivity of circulating Tumor cells derived from a colorectal Cancer patient for dual inhibition with AKT and mTOR inhibitors. Cells, 9(9)
Koch C et al (2020) Characterization of circulating breast cancer cells with tumorigenic and metastatic capacity. EMBO Mol Med 12(9):e11908
Que Z et al (2019) Establishment and characterization of a patient-derived circulating lung tumor cell line in vitro and in vivo. Cancer Cell Int 19(1):21
Yu M et al (2014) Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 345(6193):216–220
Hodgkinson CL et al (2014) Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med 20(8):897–903
Faugeroux V et al (2020) Genetic characterization of a unique neuroendocrine transdifferentiation prostate circulating tumor cell-derived eXplant model. Nat Commun 11(1):1884
Bidard FC et al (2018) Circulating Tumor cells in breast Cancer patients treated by Neoadjuvant Chemotherapy: a Meta-analysis. J Natl Cancer Inst 110(6):560–567
Effenberger KE et al (2018) Improved risk stratification by circulating Tumor Cell counts in Pancreatic Cancer. Clin Cancer Res 24(12):2844–2850
Reeh M et al (2015) Circulating Tumor cells as a biomarker for preoperative prognostic staging in patients with esophageal Cancer. Ann Surg 261(6):1124–1130
Rack B et al (2014) Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst, 106(5)
Riethdorf S et al (2017) Prognostic impact of circulating Tumor cells for breast Cancer patients treated in the Neoadjuvant Geparquattro Trial. Clin Cancer Res 23(18):5384–5393
Trapp E et al (2019) Presence of circulating Tumor cells in high-risk early breast Cancer during Follow-Up and Prognosis. J Natl Cancer Inst 111(4):380–387
Sparano J et al (2018) Association of circulating Tumor cells with late recurrence of Estrogen receptor–positive breast Cancer: a secondary analysis of a Randomized Clinical Trial. JAMA Oncol 4(12):1700–1706
Zhang L et al (2012) Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin Cancer Res 18(20):5701–5710
Lawrence R et al (2023) Circulating tumour cells for early detection of clinically relevant cancer. Nat Reviews Clin Oncol 20(7):487–500
Prognostic relevance of the HER2 status of circulating tumor cells in metastatic breast cancer patients screened for participation in the DETECT study program < sup>☆ ESMO Open, 2021. 6(6)
Agelaki S et al (2015) Efficacy of Lapatinib in Therapy-resistant HER2-Positive circulating tumor cells in metastatic breast Cancer. PLoS ONE 10(6):e0123683
Fehm T et al (2021) Abstract PD3-12: efficacy of the tyrosine kinase inhibitor lapatinib in the treatment of patients with HER2-negative metastatic breast cancer and HER2-positive circulating tumor cells - results from the randomized phase III DETECT III trial. Cancer Res 81(4Supplement):PD3–12
De Gregorio A et al (2017) Discordance in human epidermal growth factor receptor 2 (HER2) phenotype between primary tumor and circulating Tumor cells in women with HER2-Negative metastatic breast Cancer. JCO Precis Oncol 1:1–12
Nicolazzo C et al (2019) EpCAM(low) circulating Tumor cells: gold in the Waste. Dis Markers 2019:p1718920
Fehm TN et al (2018) Diagnostic leukapheresis for CTC analysis in breast cancer patients: CTC frequency, clinical experiences and recommendations for standardized reporting. Cytometry A 93(12):1213–1219
Abdo M et al (2023) Comparative evaluation of PD-L1 expression in cytology imprints, circulating tumour cells and tumour tissue in non-small cell lung cancer patients. Mol Oncol 17(5):737–746
Münsterberg J et al (2020) ALCAM contributes to brain metastasis formation in non-small-cell lung cancer through interaction with the vascular endothelium. Neuro Oncol 22(7):955–966
Yan WT et al (2017) Circulating tumor cell status monitors the treatment responses in breast cancer patients: a meta-analysis. Sci Rep 7:43464
Keup C, Kimmig R, Kasimir-Bauer S (2022) Multimodality in liquid biopsy: does a combination uncover insights undetectable in individual blood analytes? J Lab Med 46(4):255–264
Fernandez-Garcia D et al (2019) Plasma cell-free DNA (cfDNA) as a predictive and prognostic marker in patients with metastatic breast cancer. Breast Cancer Res 21(1):1–13
Davis AA et al (2019) Association of a novel circulating tumor DNA next-generating sequencing platform with circulating tumor cells (CTCs) and CTC clusters in metastatic breast cancer. Breast Cancer Res 21(1):1–8
Rossi G et al (2018) Cell-free DNA and circulating tumor cells: comprehensive liquid biopsy analysis in advanced breast cancer. Clin Cancer Res 24(3):560–568
Wang W et al (2017) Plasma cell-free DNA integrity plus circulating tumor cells: a potential biomarker of no distant metastasis breast cancer. Neoplasma 64(4):611–618
Liu HE et al (2020) Detection of EGFR mutations in cfDNA and CTCs, and comparison to Tumor tissue in Non-small-cell-lung-cancer (NSCLC) patients. Frontiers in Oncology, p 10
Welter L et al (2020) Treatment response and tumor evolution: lessons from an extended series of multianalyte liquid biopsies in a metastatic breast cancer patient. Mol Case Stud 6(6):a005819
Shishido SN et al (2022) Disease characterization in liquid biopsy from HER2-mutated, non-amplified metastatic breast cancer patients treated with neratinib. npj Breast Cancer 8(1):22
Keup C et al (2020) Multimodal targeted deep sequencing of circulating Tumor cells and matched cell-free DNA provides a more Comprehensive Tool to identify therapeutic targets in metastatic breast Cancer patients. Cancers 12(5):1084
Tzanikou E et al (2019) PIK3CA hotspot mutations in circulating tumor cells and paired circulating tumor DNA in breast cancer: a direct comparison study. Mol Oncol 13(12):2515–2530
Keup C et al (2021) Integrative statistical analyses of multiple liquid biopsy analytes in metastatic breast cancer. Genome Med 13(1):1–14
Buscail E et al (2019) High clinical value of Liquid Biopsy to detect circulating Tumor cells and Tumor exosomes in Pancreatic Ductal Adenocarcinoma patients Eligible for Up-Front surgery. Cancers 11(11):1656
Nanou A et al Tumor-derived extracellular vesicles as complementary prognostic factors to circulating Tumor cells in metastatic breast Cancer. JCO Precision Oncology, 2023(7): p. e2200372
Schneegans S et al (2020) Pre-analytical factors affecting the establishment of a single tube assay for multiparameter liquid biopsy detection in melanoma patients. Mol Oncol 14(5):1001–1015
PANCAID Consortium. PANcreatic CAncer Initial Detection via liquid biopsy (PANCAID) (2023) [Accessed : 21 August 2023]; Available from: https://pancaid-project.eu
Pantel K, Alix-Panabieres C (2010) Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med 16(9):398–406
Roeper CM et al (2023) „Liquid biopsy –schon reif für Therapieentscheidungen? best practice onkologie, : p. 1–9
Koch C et al (2020) Pre-analytical and Analytical variables of label-independent Enrichment and Automated Detection of circulating Tumor cells in Cancer patients. Cancers (Basel), 12(2)
Neves RPL et al (2021) Proficiency testing to assess Technical Performance for CTC-Processing and Detection methods in CANCER-ID. Clin Chem 67(4):631–641
Maertens Y et al (2017) Comparison of isolation platforms for detection of circulating renal cell carcinoma cells. Oncotarget 8(50):87710–87717
European Liquid Biopsy Society. European Liquid Biopsy Society (ELBS) (2023) [Accessed : 21 August 2023]; Available from: www.elbs.eu
Lampignano R et al (2020) Multicenter evaluation of circulating cell-free DNA extraction and downstream analyses for the development of standardized (pre)analytical work flows. Clin Chem 66(1):149–160
Heidrich I et al (2021) Liquid biopsies: potential and challenges. Int J Cancer 148(3):528–545
Amin MB et al (2017) The Eighth Edition AJCC Cancer staging Manual: continuing to build a bridge from a population-based to a more personalized approach to cancer staging. CA Cancer J Clin 67(2):93–99
Bardelli A, Pantel K (2017) Liquid biopsies, what we do not know (yet). Cancer Cell 31(2):172–179
Pascual J et al (2022) ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: a report from the ESMO Precision Medicine Working Group. Ann Oncol 33(8):750–768
Hofman P (2023) Implementation of the clinical practice of liquid biopsies for thoracic oncology the experience of the RespirERA university hospital institute (Nice, France). J Liquid Biopsy 1:100004
Rao A, Antonarakis ES (2019) Circulating tumor cell-based or tissue biopsy-based AR-V7 detection: which provides the greatest clinical utility? Ann Transl Med 7(Suppl 8):S354
Ignatiadis M, Sledge GW, Jeffrey SS (2021) Liquid biopsy enters the clinic — implementation issues and future challenges. Nat Reviews Clin Oncol 18(5):297–312
Bidard F-C et al (2023) Overall survival with circulating Tumor Cell Count–Driven choice of therapy in advanced breast Cancer: a Randomized Trial. J Clin Oncol, : p. JCO.23.00456.
Fehm T et al (2024) Efficacy of Lapatinib in patients with HER2-Negative metastatic breast Cancer and HER2-Positive circulating tumor cells—the DETECT III Clinical Trial. Clin Chem 70(1):307–318
Lui YY et al (2002) Predominant hematopoietic origin of cell-free DNA in plasma and serum after sex-mismatched bone marrow transplantation. Clin Chem 48(3):421–427
GUIDE.MRD Consortium. Guiding multi-modal therapies against MRD by liquid biopsies (2023) [Accessed : 21 August 2023]; Available from: https://www.guidemrd-horizon.eu
Pantel K et al (2023) 237TiP GUIDE.MRD: a Consortium guiding multi-modal therapies against minimal residual disease (MRD) by liquid biopsy to assess implementation of circulating tumor DNA (ctDNA) in clinical practice to improve patient outcomes. Ann Oncol 34:S276–S277
Connors D et al (2020) International liquid biopsy standardization alliance white paper. Crit Rev Oncol Hematol 156:103112
Jakobsen AKM, Spindler KG (2023) ctDNA-Response evaluation criteria in solid tumors - a new measure in medical oncology. Eur J Cancer 180:180–183
Acknowledgements
The Figure was created using BioRender.com.
Funding
K.P. received grant/research support from EU/IHI GUIDE.MRD (No. 101112066), EU Horizon PANCAID (No. 101096309) and ERC Grant INJURMET (No. 834974).
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
D.J.S. and S.S. reviewed the literature and wrote the original draft. All authors critically revised the original draft. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
K.P. received honoraria outside of this work from NRich, BMS, Agena, Menarini, Novartis, Sanofi, Illumina, Abcam, MSD, Eppendorf and Hummingbird.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Smit, D.J., Schneegans, S. & Pantel, K. Clinical applications of circulating tumor cells in patients with solid tumors. Clin Exp Metastasis (2024). https://doi.org/10.1007/s10585-024-10267-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10585-024-10267-5