Abstract
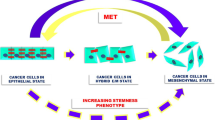
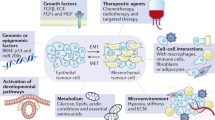
Establishing macrometastases at distant organs is a highly challenging process for cancer cells, with extremely high attrition rates. A very small percentage of disseminated cells have the ability to dynamically adapt to their changing micro-environments through reversibly switching to another phenotype, aiding metastasis. Such plasticity can be exhibited along one or more axes—epithelial–mesenchymal plasticity (EMP) and cancer stem cells (CSCs) being the two most studied, and often tacitly assumed to be synonymous. Here, we review the emerging concepts related to EMP and CSCs across multiple cancers. Both processes are multi-dimensional in nature; for instance, EMP can be defined on morphological, molecular and functional changes, which may or may not be synchronized. Similarly, self-renewal, multi-lineage potential, and resistance to anoikis and/or therapy may not all occur simultaneously in CSCs. Thus, understanding the complexity in defining EMP and CSCs is essential if we are to understand their contribution to cancer metastasis. This will require a more comprehensive understanding of the non-linearity of these processes. These processes are dynamic, reversible, and semi-independent in nature; cells traverse the inter-connected high-dimensional EMP and CSC landscapes in diverse paths, each of which may exhibit a distinct EMP-CSC coupling. Our proposed model offers a potential unifying framework for elucidating the coupled decision-making along these dimensions and highlights a key set of open questions to be answered.



Similar content being viewed by others
Data availability
Not applicable (this is a review article).
References
Gupta GP, Massagué J (2006) Cancer metastasis: building a framework. Cell 127:679–695
Celià-Terrassa T, Kang Y (2016) Distinctive properties of metastasis-initiating cells. Genes Dev 30:892–908. https://doi.org/10.1101/gad.277681.116
Celià-Terrassa T, Jolly MK (2020) Cancer stem cells and epithelial-to-mesenchymal transition in cancer metastasis. Cold Spring Harb Perspect Med 10:a036905. https://doi.org/10.1101/cshperspect.a036905
Bocci F, Levine H, Onuchic JN, Jolly MK (2019) Deciphering the dynamics of epithelial-mesenchymal transition and cancer stem cells in tumor progression. Curr Stem Cell Rep 5:11–21
Jolly MK, Ware KE, Gilja S et al (2017) EMT and MET: necessary or permissive for metastasis? Mol Oncol 11:755–769. https://doi.org/10.1002/1878-0261.12083
Pastushenko I, Blanpain C (2019) EMT transition states during tumor progression and metastasis. Trends Cell Biol 29:212–226. https://doi.org/10.1016/j.tcb.2018.12.001
Lu M, Jolly MK, Levine H et al (2013) MicroRNA-based regulation of epithelial-hybrid-mesenchymal fate determination. Proc Natl Acad Sci USA 110:18144–18149. https://doi.org/10.1073/pnas.1318192110
Pastushenko I, Brisebarre A, Sifrim A et al (2018) Identification of the tumour transition states occurring during EMT. Nature 556:463–468. https://doi.org/10.1038/s41586-018-0040-3
Huang RY-J, Wong MK, Tan TZ et al (2013) An EMT spectrum defines an anoikis-resistant and spheroidogenic intermediate mesenchymal state that is sensitive to e-cadherin restoration by a src-kinase inhibitor, saracatinib (AZD0530). Cell Death Dis 4:e915. https://doi.org/10.1038/cddis.2013.442
Tan TZ, Miow QH, Miki Y et al (2014) Epithelial-mesenchymal transition spectrum quantification and its efficacy in deciphering survival and drug responses of cancer patients. EMBO Mol Med 6:1279–1293. https://doi.org/10.15252/emmm.201404208
Carduner L, Leroy-Dudal J, Picot CR et al (2014) Ascites-induced shift along epithelial-mesenchymal spectrum in ovarian cancer cells: enhancement of their invasive behavior partly dependant on αv integrins. Clin Exp Metastas 31:675–688. https://doi.org/10.1007/s10585-014-9658-1
Simeonov KP, Byrns CN, Clark ML et al (2020) Single-cell lineage and transcriptome reconstruction of metastatic cancer reveals selection of aggressive hybrid EMT states. bioRxiv. https://doi.org/10.1101/2020.08.11.245787
Biddle A, Liang X, Gammon L et al (2011) Cancer stem cells in squamous cell carcinoma switch between two distinct phenotypes that are preferentially migratory or proliferative. Cancer Res 71:5317–5326. https://doi.org/10.1158/0008-5472.CAN-11-1059
Biswas K, Jolly MK, Ghosh A (2019) Stability and mean residence times for hybrid epithelial/mesenchymal phenotype. Phys Biol 16:025003. https://doi.org/10.1088/1478-3975/aaf7b7
Gupta PB, Fillmore CM, Jiang G et al (2011) Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell 146:633–644. https://doi.org/10.1016/j.cell.2011.07.026
Tripathi S, Chakraborty P, Levine H, Jolly MK (2020) A mechanism for epithelial-mesenchymal heterogeneity in a population of cancer cells. PLoS Comput Biol 16:e1007619. https://doi.org/10.1101/592691
Katsuno Y, Meyer DS, Zhang Z et al (2019) Chronic TGF-β exposure drives stabilized EMT, tumor stemness, and cancer drug resistance with vulnerability to bitopic mTOR inhibition. Sci Signal 12:eaau8544. https://doi.org/10.1126/scisignal.aau8544
Jia W, Deshmukh A, Mani SA et al (2019) A possible role for epigenetic feedback regulation in the dynamics of the Epithelial-Mesenchymal Transition (EMT). Phys Biol 16:066004. https://doi.org/10.1088/1478-3975/ab34df
Jia W, Tripathi S, Chakraborty P et al (2020) Epigenetic feedback and stochastic partitioning during cell division can drive resistance to EMT. Oncotarget 11:2611–2624. https://doi.org/10.18632/oncotarget.27651
Biddle A, Gammon L, Liang X et al (2016) Phenotypic plasticity determines cancer stem cell therapeutic resistance in oral squamous cell carcinoma. EBioMedicine 4:138–145. https://doi.org/10.1016/j.ebiom.2016.01.007
Celià-Terrassa T, Bastian C, Liu DD et al (2018) Hysteresis control of epithelial-mesenchymal transition dynamics conveys a distinct program with enhanced metastatic ability. Nat Commun 9:5005. https://doi.org/10.1038/s41467-018-07538-7
Karacosta LG, Anchang B, Ignatiadis N et al (2019) Mapping lung cancer epithelial-mesenchymal transition states and trajectories with single-cell resolution. Nat Commun 10:5587. https://doi.org/10.1101/570341
Stylianou N, Lehman ML, Wang C et al (2019) A molecular portrait of epithelial–mesenchymal plasticity in prostate cancer associated with clinical outcome. Oncogene 38:913–934. https://doi.org/10.1038/s41388-018-0488-5
Schmidt JM, Panzilius E, Bartsch HS et al (2015) Stem-cell-like properties and epithelial plasticity arise as stable traits after transient twist1 activation. Cell Rep 10:131–139. https://doi.org/10.1016/j.celrep.2014.12.032
Cheung KJ, Ewald AJ (2014) Illuminating breast cancer invasion: diverse roles for cell-cell interactions. Curr Opin Cell Biol 30:99–111. https://doi.org/10.1016/j.ceb.2014.07.003
Schaeffer D, Somarelli JA, Hanna G et al (2014) Cellular migration and invasion uncoupled: increased migration is not an inexorable consequence of epithelial-to-mesenchymal transition. Mol Cell Biol 34:3486–3499. https://doi.org/10.1128/MCB.00694-14
Devaraj V, Bose B (2019) Morphological state transition dynamics in EGF-induced epithelial to mesenchymal transition. J Clin Med 8:911. https://doi.org/10.3390/jcm8070911
Foroutan M, Bhuva DD, Lyu R et al (2018) Single sample scoring of molecular phenotypes. BMC Bioinform 19:404. https://doi.org/10.1186/s12859-018-2435-4
Puram SV, Tirosh I, Parikh AS et al (2017) Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell 171:1611–1624. https://doi.org/10.1016/j.cell.2017.10.044
Cheung KJ, Padmanaban V, Silvestri V et al (2016) Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc Natl Acad Sci USA 113:E854–E863. https://doi.org/10.1073/pnas.1508541113
Tripathi S, Jolly MK, Woodward WA et al (2018) Analysis of hierarchical organization in gene expression networks reveals underlying principles of collective tumor cell dissemination and metastatic aggressiveness of inflammatory breast cancer. Front Oncol 8:244. https://doi.org/10.1101/204388
Subbalakshmi AR, Sahoo S, Biswas K, Jolly MK (2021) A computational systems biology approach identifies SLUG as a mediator of partial Epithelial-Mesenchymal Transition (EMT). Cells Tissues Organs. https://doi.org/10.1159/000512520
Steinbichler TB, Dudas J, Ingruber J et al (2020) Slug is a surrogate marker of Epithelial to Mesenchymal Transition (EMT) in head and neck cancer. J Clin Med 9:2061. https://doi.org/10.3390/jcm9072061
Leroy P, Mostov KE (2007) Slug is required for cell survival during partial epithelial-mesenchymal transition of HGF-induced tubulogenesis. J Cell Sci 18:1943–1952. https://doi.org/10.1091/mbc.E06
Schinke H, Pan M, Akyol M et al (2020) Partial epithelial-to-mesenchymal transition is prognostic and associates with slug in head and neck cancer. bioRxiv 346692
Karaosmanoğlu O, Banerjee S, Sivas H (2018) Identification of biomarkers associated with partial epithelial to mesenchymal transition in the secretome of slug over-expressing hepatocellular carcinoma cells. Cell Oncol 41:439–453. https://doi.org/10.1007/s13402-018-0384-6
Sterneck E, Poria DK, Balamurugan K (2020) Slug and E-cadherin: stealth accomplices? Front Mol Biosci 7:138. https://doi.org/10.3389/fmolb.2020.00138
Andriani F, Bertolini G, Facchinetti F et al (2016) Conversion to stem-cell state in response to microenvironmental cues is regulated by balance between epithelial and mesenchymal features in lung cancer cells. Mol Oncol 10:253–271. https://doi.org/10.1016/j.molonc.2015.10.002
Hong T, Watanabe K, Ta CH et al (2015) An Ovol2-Zeb1 mutual inhibitory circuit governs bidirectional and multi-step transition between epithelial and mesenchymal states. PLOS Comput Biol 11:e1004569. https://doi.org/10.1371/journal.pcbi.1004569
Bocci F, Tripathi SC, Vilchez Mercedes SA et al (2019) NRF2 activates a partial epithelial-mesenchymal transition and is maximally present in a hybrid epithelial/mesenchymal phenotype. Integr Biol 11:251–263. https://doi.org/10.1093/intbio/zyz021
Subbalakshmi AR, Kundnani D, Biswas K et al (2020) NFATc acts as a non-canonical phenotypic stability factor for a hybrid epithelial/mesenchymal phenotype. Front Oncol 10:1794. https://doi.org/10.3389/fonc.2020.553342
Sampson VB, David JM, Puig I et al (2014) Wilms’ tumor protein induces an epithelial-mesenchymal hybrid differentiation state in clear cell renal cell carcinoma. PLoS ONE 9:e102041. https://doi.org/10.1371/journal.pone.0102041
Silveira DA, Gupta S, Mombach JCM (2020) Systems biology approach suggests new miRNAs as phenotypic stability factors in the epithelial–mesenchymal transition. J R Soc Interface 17:20200693. https://doi.org/10.1098/rsif.2020.0693
Dang TT, Esparza MA, Maine EA et al (2015) ∆Np63α promotes breast cancer cell motility through the selective activation of components of the Epithelial-to-Mesenchymal Transition program. Cancer Res 75:3925–3935. https://doi.org/10.1158/0008-5472.CAN-14-3363
Mandal M, Ghosh B, Anura A et al (2016) Modeling continuum of epithelial mesenchymal transition plasticity. Integr Biol 8:167–176. https://doi.org/10.1039/C5IB00219B
Jolly MK, Tripathi SC, Jia D et al (2016) Stability of the hybrid epithelial/mesenchymal phentoype. Oncotarget 7:27067–27084
Varankar SS, Kamble SS, Mali AM et al (2020) Functional balance between TCF21-Slug defines cellular plasticity and sub-classes in high-grade serous ovarian cancer. Carcinogenesis 41:515–526. https://doi.org/10.1093/carcin/bgz119
Subbalakshmi AR, Ashraf B, Jolly MK (2021) Biophysical and biochemical attributes of hybrid epithelial/mesenchymal phenotypes. Preprints 2021080453. https://doi.org/10.20944/preprints202108.0451.v1
Margaron Y, Nagai T, Guyon L et al (2019) Biophysical properties of intermediate states of EMT outperform both epithelial and mesenchymal states. bioRxiv. https://doi.org/10.1101/797654
Aponte PM, Caicedo A (2017) Stemness in cancer: stem cells, cancer stem cells, and their microenvironment. Stem Cells Int 2017:5619472. https://doi.org/10.1155/2017/5619472
Malta TM, Sokolov A, Gentles AJ et al (2018) Machine learning identifies stemness features associated with oncogenic dedifferentiation. Cell 173:338–354. https://doi.org/10.1016/j.cell.2018.03.034
Grün D, Muraro MJ, Boisset JC et al (2016) De novo prediction of stem cell identity using single-cell transcriptome data. Cell Stem Cell 19:266–277. https://doi.org/10.1016/j.stem.2016.05.010
Gulati GS, Sikandar SS, Wesche DJ et al (2020) Single-cell transcriptional diversity is a hallmark of developmental potential. Science 367:405–411. https://doi.org/10.1126/science.aax0249
Efremov YR, Proskurina AS, Potter EA et al (2018) Cancer stem cells: emergent nature of tumor emergency. Front Genet 9:544. https://doi.org/10.3389/fgene.2018.00544
Frisch SM, Schaller M, Cieply B (2013) Mechanisms that link the oncogenic epithelial- mesenchymal transition to suppression of anoikis. J Cell Sci 126:21–29. https://doi.org/10.1242/jcs.120907
Ho MM, Ng AV, Lam S, Hung JY (2007) Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res 67:4827–4833. https://doi.org/10.1158/0008-5472.CAN-06-3557
Shi Y, Fu X, Hua Y et al (2012) The side population in human lung cancer cell line NCI-H460 is enriched in stem-like cancer cells. PLoS ONE 7:e33358. https://doi.org/10.1371/journal.pone.0033358
Ray A, Slama ZM, Morford RK et al (2017) Enhanced directional migration of cancer stem cells in 3D aligned collagen matrices. Biophys J 112:1023–1036. https://doi.org/10.1016/j.bpj.2017.01.007
Bertolini G, Roz L, Perego P et al (2009) Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci USA 106:16281–16286. https://doi.org/10.1073/pnas.0905653106
Terris B, Cavard C, Perret C (2010) EpCAM, a new marker for cancer stem cells in hepatocellular carcinoma. J Hepatol 52:280–281. https://doi.org/10.1016/j.jhep.2009.10.026
Liu S, Cong Y, Wang D et al (2014) Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep 2:78–91. https://doi.org/10.1016/j.stemcr.2013.11.009
Iliopoulos D, Hirsch HA, Wang G, Struhl K (2011) Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc Natl Acad Sci USA 108:1397–1402. https://doi.org/10.1073/pnas.1018898108
Bierie B, Pierce SE, Kroeger C et al (2017) Integrin-β4 identifies cancer stem cell-enriched populations of partially mesenchymal carcinoma cells. Proc Natl Acad Sci USA 114:E2337-2346. https://doi.org/10.1073/pnas.1618298114
Al-Hajj M, Wicha MS, Benito-Hernandez A et al (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100:3983–3988. https://doi.org/10.1073/pnas.0530291100
Rasti A, Mehrazma M, Madjd Z et al (2018) Co-expression of cancer stem cell markers OCT4 and NANOG predicts poor prognosis in renal cell carcinomas. Sci Rep 8:11739. https://doi.org/10.1038/s41598-018-30168-4
Takeda K, Mizushima T, Yokoyama Y et al (2018) Sox2 is associated with cancer stem-like properties in colorectal cancer. Sci Rep 8:17639. https://doi.org/10.1038/s41598-018-36251-0
Yang L, Shi P, Zhao G et al (2020) Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther 5:8. https://doi.org/10.1038/s41392-020-0110-5
Neelakantan D, Zhou H, Oliphant MUJ et al (2017) EMT cells increase breast cancer metastasis via paracrine GLI activation in neighbouring tumour cells. Nat Commun 8:15773. https://doi.org/10.1038/ncomms15773
Jolly MK, Preca B-T, Tripathi SC et al (2018) Interconnected feedback loops among ESRP1, HAS2, and CD44 regulate epithelial-mesenchymal plasticity in cancer. APL Bioeng 2:031908. https://doi.org/10.1063/1.5024874
Bocci F, Gearhart-Serna L, Boareto M et al (2019) Toward understanding cancer stem cell heterogeneity in the tumor microenvironment. Proc Natl Acad Sci USA 116:148–157. https://doi.org/10.1073/pnas.1815345116
Yang G, Quan Y, Wang W et al (2012) Dynamic equilibrium between cancer stem cells and non-stem cancer cells in human SW620 and MCF-7 cancer cell populations. Br J Cancer 106:1512–1519. https://doi.org/10.1038/bjc.2012.126
Weiswald L-B, Bellet D, Dangles-Marie V (2015) Spherical cancer models in tumor biology. Neoplasia 1:1–15. https://doi.org/10.1016/j.neo.2014.12.004
Valent P, Bonnet D, De Maria R et al (2012) Cancer stem cell definitions and terminology: the devil is in the details. Nat Rev Cancer 12:767–775. https://doi.org/10.1038/nrc3368
Castelli V, Giordano A, Benedetti E et al (2021) The great escape: the power of cancer stem cells to evade programmed cell death. Cancers 13:328. https://doi.org/10.3390/cancers13020328
Yang J, Antin P, Berx G et al (2020) Guidelines and definitions for research on epithelial–mesenchymal transition. Nat Rev Mol Cell Biol 21:341–352. https://doi.org/10.1038/s41580-020-0237-9
Mani SA, Guo W, Liao M-J et al (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133:704–715. https://doi.org/10.1016/j.cell.2008.03.027
Morel A-P, Lièvre M, Thomas C et al (2008) Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE 3:e2888. https://doi.org/10.1371/journal.pone.0002888
Scheel C, Weinberg RA (2012) Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Cancer Biol 22:396–403. https://doi.org/10.1016/j.semcancer.2012.04.001
Grosse-Wilde A, Fouquier d’ Herouei A, McIntosh E et al (2015) Stemness of the hybrid epithelial/mesenchymal state in breast cancer and it. PLoS ONE 10:e0126522. https://doi.org/10.1371/journal.pone.0126522
Jolly MK, Huang B, Lu M et al (2014) Towards elucidating the connection between epithelial-mesenchymal transitions and stemness. J R Soc Interface 11:20140962. https://doi.org/10.1098/rsif.2014.0962
Colacino JA, Azizi E, Brooks MD et al (2018) Heterogeneity of human breast stem and progenitor cells as revelaed by transcriptional profiling. Stem Cell Rep 10:1596–1609. https://doi.org/10.1016/j.stemcr.2016.05.008
Ginestier C, Hur MH, Charafe-Jauffret E et al (2007) ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1:555–567. https://doi.org/10.1016/j.stem.2007.08.014
Goldman A, Majumder B, Dhawan A et al (2015) Temporally sequenced anticancer drugs overcome adaptive resistance by targeting a vulnerable chemotherapy-induced phenotypic transition. Nat Commun 6:6139. https://doi.org/10.1038/ncomms7139
Kröger C, Afeyan A, Mraz J et al (2019) Acquisition of a hybrid E/M state is essential for tumorigenicity of basal breast cancer cells. Proc Natl Acad Sci USA 116:7353–7362. https://doi.org/10.1073/pnas.1812876116
Shi R, Liu L, Wang F et al (2020) Downregulation of cytokeratin 18 induces cellular partial EMT and stemness through increasing EpCAM expression in breast cancer. Cell Signal 76:109810. https://doi.org/10.1016/j.cellsig.2020.109810
Quan Q, Wang X, Lu C et al (2020) Cancer stem-like cells with hybrid epithelial/mesenchymal phenotype leading the collective invasion. Cancer Sci 111:467–476. https://doi.org/10.1111/cas.14285
Eramo A, Lotti F, Sette G et al (2008) Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ 15:504–514. https://doi.org/10.1038/sj.cdd.4402283
Schliekelman MJ, Taguchi A, Zhu J et al (2015) Molecular portraits of epithelial, mesenchymal, and hybrid states in lung adenocarcinoma and their relevance to survival. Cancer Res 75:1789–1800. https://doi.org/10.1158/0008-5472.CAN-14-2535
George JT, Jolly MK, Xu S et al (2017) Survival outcomes in cancer patients predicted by a partial EMT gene expression scoring metric. Cancer Res 77:6415–6428. https://doi.org/10.1158/0008-5472.CAN-16-3521
Wang H, Zhang H, Tang L et al (2013) Resveratrol inhibits TGF-β1-induced epithelial-to-mesenchymal transition and suppresses lung cancer invasion and metastasis. Toxicology 303:139–146. https://doi.org/10.1016/j.tox.2012.09.017
Tièche CC, Gao Y, Bührer ED et al (2018) Tumor initiation capacity and therapy resistance are differential features of EMT-related subpopulations in the NSCLC cell line A549. Neoplasia 21:185–196. https://doi.org/10.1016/j.neo.2018.09.008
Jia D, George JT, Tripathi SC et al (2019) Testing the gene expression classification of the EMT spectrum. Phys Biol 16:025002. https://doi.org/10.1088/1478-3975/aaf8d4
Moltzahn F, Thalmann GN (2013) Cancer stem cells in prostate cancer. Transl Androl Urol 2:242–253. https://doi.org/10.3978/j.issn.2223-4683.2013.09.06
Li H, Chen X, Calhoun-Davis T et al (2008) PC3 human prostate carcinoma cell holoclones contain self-renewing tumor-initiating cells. Cancer Res 68:1820–1825. https://doi.org/10.1158/0008-5472.CAN-07-5878
Celià-Terrassa T, Meca-Cortés Ó, Mateo F et al (2012) Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. J Clin Invest 122:1849–1868. https://doi.org/10.1172/JCI59218
Padmanaban V, Krol I, Suhail Y et al (2019) E-cadherin is required for metastasis in multiple models of breast cancer. Nature 573:439–444. https://doi.org/10.1038/s41586-019-1526-3
Cook DP, Vanderhyden BC (2020) Context specificity of the EMT transcriptional response. Nat Commun 11:2142. https://doi.org/10.1038/s41467-020-16066-2
Roca H, Hernandez J, Weidner S et al (2013) Transcription factors OVOL1 and OVOL2 induce the mesenchymal to epithelial transition in human cancer. PLoS ONE 8:e76773. https://doi.org/10.1371/journal.pone.0076773
Ruscetti M, Dadashian EL, Guo W et al (2016) HDAC inhibition impedes epithelial-mesenchymal plasticity and suppresses metastatic, castration-resistant prostate cancer. Oncogene 35:3781–3795. https://doi.org/10.1038/onc.2015.444
Pastushenko I, Mauri F, Song Y et al (2021) Fat1 deletion promotes hybrid EMT state, tumour stemness and metastasis. Nature 589:448–455. https://doi.org/10.1038/s41586-020-03046-1
Youssef G, Gammon L, Ambler L et al (2020) Disseminating cells in human tumous acquire an EMT stem cell state that is predictive of metastasis. bioRxiv. https://doi.org/10.1101/2020.04.07.029009
McFaline-Figueroa JL, Hill AJ, Qiu X et al (2019) A pooled single-cell genetic screen identifies regulatory checkpoints in the continuum of the epithelial-to-mesenchymal transition. Nat Genet 51:1389–1398. https://doi.org/10.1038/s41588-019-0489-5
Jia D, Jolly MK, Kulkarni P, Levine H (2017) Phenotypic plasticity and cell fate decisions in cancer : insights from dynamical systems theory. Cancers 9:E70. https://doi.org/10.3390/cancers9070070
Cantelli G, Orgaz JL, Rodriguez-Hernandez I et al (2015) TGF-β-induced transcription sustains amoeboid melanoma migration and dissemination. Curr Biol 25:2899–2914. https://doi.org/10.1016/j.cub.2015.09.054
Rodriguez-Hernandez I, Maiques O, Kohlhammer L et al (2020) WNT11-FZD7-DAAM1 signalling supports tumour initiating abilities and melanoma amoeboid invasion. Nat Commun 11:5315. https://doi.org/10.1038/s41467-020-18951-2
Taddei ML, Giannoni E, Morandi A et al (2014) Mesenchymal to amoeboid transition is associated with stem-like features of melanoma cells. Cell Commun Signal 12:24. https://doi.org/10.1186/1478-811X-12-24
Ben-Porath I, Thomson MWM, Carey VJVVJ et al (2008) An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet 40:499–507. https://doi.org/10.1038/ng.127
Wagner DE, Klein AM (2020) Lineage tracing meets single-cell omics: opportunities and challenges. Nat Rev Genet 21:410–427. https://doi.org/10.1038/s41576-020-0223-2
Chauhan L, Ram U, Hari K, Jolly MK (2021) Topological signatures in regulatory network enable phenotypic heterogeneity in small cell lung cancer. Elife 10:e64522
Pillai M, Jolly MK (2021) Systems-level network modeling deciphers the master regulators of phenotypic plasticity and heterogeneity in melanoma. bioRxiv. https://doi.org/10.1101/2021.03.11.434533
Pasani S, Sahoo S, Jolly MK (2021) Hybrid E/M phenotype(s) and stemness: a mechanistic connection embedded in network topology. J Clin Med 10:60. https://doi.org/10.1101/2020.10.18.341271
Lambert AW, Weinberg RA (2021) Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat Rev Cancer. https://doi.org/10.1038/s41568-021-00332-6
Kahounová Z, Remšík J, Fedr R et al (2020) Slug-expressing mouse prostate epithelial cells have increased stem cell potential. Stem Cell Res 46:101844. https://doi.org/10.1016/j.scr.2020.101844
Luanpitpong S, Li J, Manke A et al (2016) SLUG is required for SOX9 stabilization and functions to promote cancer stem cells and metastasis in human lung carcinoma. Oncogene 35(22):2824–2833. https://doi.org/10.1038/onc.2015.351
Acknowledgements
MKJ was supported by Ramanujan Fellowship (SB/S2/RJN-049/2018) awarded by Science and Engineering Research Board, Department of Science and Technology, Government of India; and by InfoSys Foundation, Bangalore. ASD acknowledges support from Prime Ministers’ Research Fellowship (PMRF). AB is supported by Animal Free Research UK.
Author information
Authors and Affiliations
Contributions
MKJ and AB conceptualized and edited the review; SS, BA and ASD prepared the first version and associated artwork. All authors contributed to writing the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sahoo, S., Ashraf, B., Duddu, A.S. et al. Interconnected high-dimensional landscapes of epithelial–mesenchymal plasticity and stemness in cancer. Clin Exp Metastasis 39, 279–290 (2022). https://doi.org/10.1007/s10585-021-10139-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-021-10139-2