Abstract
To determine the effect of bipolar cooled radiofrequency ablation (BCRF) on bone and tumour in a lapine pathologic femoral model. Under institutional approval, twelve New Zealand white rabbits received a single femoral injection of VX2 carcinoma cells (day 0). The rabbit femora, (n = 24), were block-randomized into four experimental groups: tumour-bearing radiofrequency ablation (RFA) treated, healthy bone RFA treated, tumour-bearing shams and healthy bone shams (n = 6 per group). 15 min of thermally regulated (65 °C) BCRF was applied at day 14. Pre- and post-treatment MR imaging was performed and repeated at day 28 prior to euthanasia. Histologic evaluation was used to determine treatment effect on tumour and bone tissue. A thirteenth injected rabbit served as a histologic control (no BCRF electrode placement). Large volumes (12.9 ± 5.5 cm3) of thermal ablation were achieved. An eight-fold reduction in tumour growth resulted in RFA treated animals compared to tumour-bearing sham controls (p < 0.001). Osteolysis was controlled in the tumour-treated group. Therapeutic effects were best imaged using MR contrast-enhanced SPoiled Gradient Recalled (SPGR) sequences. Osteoclasts and osteoblasts were observed to be sensitive to BCRF but osteocytes were more resilient. A small number of tumour cells within BCRF treated regions appeared viable post treatment. New bone formation was stimulated in the periphery of the targeted BCRF treatment zone. Structurally large VX2 tumour volumes within bone were successfully ablated with BCRF, stimulating new bone formation in the treatment periphery, although viable appearing osteocytes and tumour cells were observed in some treated regions.
Similar content being viewed by others
Introduction
Advances in bone-targeted radiofrequency ablation (RFA) aim to improve quality of life for many patients who suffer from skeletal metastases. Conventional therapy for bone metastases includes systemic bisphosphonates [1], radiation therapy and in carefully and appropriately selected patients, interventional radiology and/or surgical approaches. RFA has been used clinically to treat painful non-mechanically significant primary and secondary skeletal lesions [2–7]. However, RFA treatment of structurally large bone lesions remains a challenge [8]. RFA functions by directing alternating electrical current to locally excite ionic cellular components, relying on successful heat conduction and completion of an electric circuit. The application of conventional RFA has been successful in treating soft tissues and structurally confined, small primary bone tumours (i.e. osteoid osteomas) [9, 10]. However, there are limitations to conventional electrode applicators, historically designed for soft tissues when considering their potential application to larger structural diseased human bone. Existing challenges include therapeutically small zones of ablation, inconsistent regions of thermal effects, tissue carbonization and desiccation [11]. A challenge for RFA application in larger osseous lesions representative of skeletal metastasis is completing an electrical circuit in bone and achieving sufficient conduction, which depends on the thermal and electrical properties of bone [12]. There is limited scientific literature concerning evaluation of RFA for bone tumours, in particular for structurally large bone lesions [13–15]. Advancements in RF electrode applicator design have enabled the generation of structurally large ablation volumes within skeletal tissue, however the impact of RFA on large areas of tumour involved bone have not been well described. This study aims to determine the effect of bipolar cooled radiofrequency ablation (BCRF) on bone and tumour cells in a diseased lapine model, and to compare MR imaging effects in reflecting histological ablation within the diseased skeleton.
Materials and methods
Experimental design
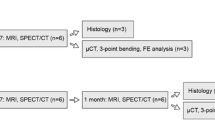
Institutional animal care ethics approval was obtained for the study. Twelve New Zealand white (NZW) rabbits underwent an intramedullary injection of VX2 tumour cells into one randomly selected femur. Bone tumours developed over 14 days following cell inoculation. A thirteenth animal (also injected with VX2 tumour cells) was sacrificed as a control for histology on day 14 and MR scanning to select the sequences for bone tumour radiologic evaluation that best highlight tumour burden.
In the remaining twelve animals, a block randomization protocol assigned animals to one of four experimental groups: tumour-bearing RFA treated, healthy RFA treated, tumour-bearing sham and healthy sham groups (n = 6 per group). RFA or sham treatments were applied on day 14. Treatment effects were evaluated by MRI performed on day 28. Animals were euthanized and bone tissues harvested (Fig. 1). This time point following RFA was selected based on our preliminary work and a literature review of published VX2 studies in rabbit long bones [16–20].
Femoral VX2 tumour cell injection
Anesthesia in each rabbit was induced by intra-muscular injection of ketamine (15 mg/kg) and xylazine (2 mg/kg) with maintenance inhalation anesthesia [2–3 % isoflurane in oxygen (2 L/min)]. A small incision (2 cm) was made at the distal end of the femur through which a small hole was drilled (1.2 mm diameter) for tumour cell injection (200 μl of VX2 cell suspension (~5x103/μl) using a 25 gauge needle). The drill hole was placed at the distal femoral intercondylar notch. Following cell injection, the drill hole was sealed with bone wax and the incision suture closed.
Bone-targeted radiofrequency ablation
A bipolar cooled radiofrequency (BCRF) applicator (OsteoCool®, Baylis Medical Company, Mississauga, ON, Canada) was used to administer the procedure. The BCRF system included a 17G (1.518 mm outer diameter) electrode applicator and a RF generator that delivered electrical energy at a frequency of 460 kHz (maximum hardware output power of 50 W). The system used a default controlled ramp rate of 10 °C/min such that a final temperature of 65 °C was reached after approximately 3.5 min. A thermocouple at the tip of the applicator continuously measured the temperature throughout the procedure. This temperature feedback maintained the applicator tip at the set temperature and prevented charring. On the day of RFA therapy (14 days after cell injection), each rabbit was anesthetized, and the intercondylar notch drill hole accessed to position the RFA applicator into the femoral medullary cavity. In the tumour group, pre-treatment MRI helped locate the tumour within the femur and measurements based on the images, guided subsequent electrode applicator placement. Under fluoroscopy (BV Pulsera, Philips, Model # MD0709BRM, Sarronno, Italy), the electrode applicator was positioned into the tumour residing within the intra-medullary cavity, with the radiopaque band (located at the proximal end of the active tip) of the BCRF electrode applicator positioned approximately at the distal edge of the tumour. To monitor heat effects outside the bone, a second temperature sensor probe (22G) was placed exterior to the surface of the femur and near the ablation electrode applicator tip (Fig. 2). The treatment duration was 15 min (including ramp up time) with a 65 °C set temperature. For sham control samples, the electrode applicator was placed and no electrical current was applied. The heart rate and oxygen levels of each rabbit were monitored during the procedure. After the tumour cell injection and the RFA procedure, the animals were observed daily to assess their overall wellbeing and their level of lower extremity load bearing as a qualitative measure of treatment safety.
Magnetic resonance imaging
Imaging was performed on rabbit femora under anesthesia prior to and immediately following RFA treatment (day 14) as well as immediately prior to animal sacrifice (day 28) on a 3.0T GE scanner (GE Healthcare, Milwaukee, WI) using a 5″ surface coil. Images were acquired using standard 3D-SPoiled Gradient Recalled (SPGR) and 3D-fast imaging employing steady state acquisition (FIESTA) sequences. A typical field of view of 16 cm (matrix = 256 × 256), slice thickness of 3 mm, TR/TE 8.3/3.1 ms and flip angle of 55 was used for the FIESTA sequences. A typical field of view of 16 cm (matrix = 512 × 512), slice thickness of 3 mm, TR/TE 4.4/1.6 ms and flip angle of 30 was used for the SPGR sequences. Gd-DTPA contrast agent (0.1 mmol/kg) was injected intravenously and post contrast images were acquired. All images were analyzed to quantify surface area and volume measurements of the tumour and to assess the ablation zone (including the peripheral rim) by manual segmentation of the region of effect (Amira 5.2 software, Visage Imaging GmbH, Germany). Results were then compared to histology images (described below), to confirm the zone of ablation and qualitatively assess the ease of detection of the ablated regions using MRI.
Histology
The femora containing tumour and surrounding thigh soft tissues were harvested post sacrifice. Specimens were fixed in 10 % neutral buffered formalin. To facilitate EDTA decalcification [21], the femora were cut transversely at the proximal ends (i.e. distant to tumour and therapy region). Hematoxylin and eosin (H&E) staining was used to assess the osseous ablation zone. Tartrate-resistant acid phosphatase (TRAP) staining was used to identify osteoclast activity in each of the diseased and healthy samples. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was used to identify apoptotic cells in bone. Cell death was determined by counting the number of cells containing nuclei with apoptotic morphology that were also stained dark brown with 3,3′-Diaminobenzidine (DAB). A positive pixel count algorithm was used. Finally, AE1/AE3 cytokeratin staining of cells of epithelial origin was used to determine VX2 tumour cell necrosis. Cells with melted, indistinct membrane and coagulated cytoplasm and nuclei were counted as treated and dead, while intact, plump cells with clear healthy morphology were considered unaffected.
Study design and analysis
Sample size was determined using the web-based Harvard Sample Size Calculator for a parallel study, with standard deviation and difference-in-means input values determined via measurements from the size of ablation zone (i.e. volume of an ovoid) of pilot experiment’s lesions. Statistical analysis of the two treatment parallel-design study was conducted using a Student’s t test to compare pairs of independent groups by means of PASW software (Version 18.0, Chicago, IL, USA).
Results
VX2 tumour growth and application of RFA
VX2 tumours were generated in all injected rabbit femora. Manual segmentation of the MR images revealed generated femoral tumours ranging from 0.9 to 1.9 cm3 in volume (average 1.4 ± 0.5 cm3) at day 14 (Table 1). RFA treatment was technically successful without any device-related issues or disruptions. The RF generator was stable through the procedure delivering a consistent energy output of 5–10 W to treated femora over the prescribed 15 min.
Clinical and imaged based RFA effects
Ablation size
The MR images of the RFA treated femora consistently revealed zones of ablation that affected intramedullary and cortical bone regions as well as the surrounding soft tissues of the thigh. Ablation volumes of tumour bearing and healthy bones based on all MR sequences confirmed an average of 12.9 ± 5.5 cm3 of treated volume (with an average length and diameter of 3.1 ± 0.3 and 1.5 ± 0.3 cm respectively) including both bone and adjacent soft tissues (Table 2). [Note: the size of ablation was relatively large compared to the rabbit anatomy as the device used was designed for human application and anticipated human volumes].
Effect of treatment on the tumour in bone
The large zone of ablation created did not always correspond with the tumour distribution along the femur; viable tumour cells remained outside the treatment volume in all samples (Fig. 3). In two of six RFA treated tumour bearing animals, a small number of viable tumour cells were observed histologically (H&E and sAE1/AE3) within the RFA treated region. The size of tumour was reduced in RFA treated animals with an eight-fold reduction in viable tumour volume at day 28 compared to the untreated tumour bearing RFA sham group (p < 0.001) (Table 1; Fig. 4). Tumour growth in the RFA untreated sham group extended beyond the cortex at the distal femoral end, forming new masses typically in the surrounding soft tissues.
AE1/AE3 staining and MR images of tumour-bearing femora. a and b Tumour growth on day 14, c Tumour growth on day 28 (without treatment). (broad pointed down arrow) Demonstrates the VX2 tumour cells which partially occupy the bone on day 14 (a), and almost completely occupy the bone on day 28 (c). d Treated tumour-bearing femur showing the RFA region of effect (narrow pointed down arrow) that has ablated the tumour within RF treatment zone. e High magnification representation of untreated VX2 cells and f high magnification of a treated VX2 zone. g and h MR images of the femora highlighting untreated and RF treated tumour respectively
Clinical outcomes
The rabbits were clinically assessed before and after tumour cell injection as well as post RFA treatment. Special attention was paid to weight bearing of the injected/treated leg. All rabbits tolerated the tumour injection well and did not show any signs of lameness. After the RFA treatment, the rabbits showed low levels of pain (decreased appetite and movement), which was successfully treated with subcutaneous injection of buprenorphine over 2 days. Only one rabbit showed additional signs of a slight hind limb lameness, which may have been due to the treatment damaging femoral nerve branches. This was diagnosed after a neurological examination of the rabbit [22]. The mild degree of lameness did not interfere with the overall wellbeing of the rabbit and therefore it was not excluded from the study.
MRI
Tumour tissue was detectable prior to RF ablation using all four imaging sequences. The tumour features were most easily discernible using Gd-enhanced SPGR sequences. Post ablation however, it was more difficult to distinguish the effects of ablation and necrosis on bone and tumour from residual or live tumour using MR imaging alone. As such live tumour was selected on the images after comparison with histological evidence. The ablation zone was best distinguished from remaining tumour and healthy tissues using contrast enhanced MR imaging (Gd-SPGR and Gd-FIESTA). Since MRI does not provide any information regarding mineralized tissues, the cortical femoral shell appeared intact in these images, without any signal change among groups (Fig. 4). A significantly higher volume of tumour was detected in the sham group compared to the treated group (average of 2.58 ± 0.00 vs. 0.35 ± 0.09 cm3 respectively, p < 0.001). The ablation volume was 1000× greater than the volume of injury induced by electrode applicator placement in the sham group (p < 0.001).
Histologic effects of RFA on bone
H&E staining demonstrated enhancement in the treatment region of the experimental group (Fig. 5). Coagulative necrosis was evident in the medullary cavity of these treated regions surrounded by areas of fibrous tissue formation (healthy group) and VX2 cells (tumour-bearing group) at the periphery of the RFA treatment zone. TUNEL staining confirmed RFA apoptotic effects on bone cells in the cortex as well as along the periosteum and endosteum of the cortex.
Newly formed bone (green outline) in a healthy treated femur. a Low magnification Hematoxylin and eosin (H&E) stain with enhanced staining in the treatment region, new bone at the edges of the treatment region (arrow) and the electrode applicator track (filled arrow). b Corresponding high magnification view of the new trabecular-like bone and the abundant osteoblasts (short arrows). (Color figure online)
As expected, tumour-bearing femora exhibited a significantly elevated level of osteoclastic activity when compared to the healthy control femora (Fig. 6). Osteoclasts were observed in high concentrations directly adjacent to tumour tissue. In addition to an overall increased number of large (≥3 nuclei, p < 0.001) and small (<3 nuclei, p < 0.005) osteoclasts, tumour-bearing femora exhibited a higher percentage of large to small osteoclasts when compared to healthy control femora. TRAP staining demonstrated a significant reduction in osteoclast counts (both large and small) with RFA treatment compared to the respective untreated control groups (Table 3).
Tartrate-resistant acid phosphatase (TRAP) staining, demonstrating osteoclasts activity in a tumour (T)-bearing untreated sham control, b treated tumour (TT)-bearing femur, c healthy sham (with healthy bone marrow, BM), and d Treated healthy femur (with RF treated bone marrow, TBM). Osteoclasts are notably increased in the untreated tumour group
TUNEL staining showed a high number of apoptotic bone-lining osteoblasts in the RFA treated femora (both tumour bearing and healthy groups). Unstained cells demonstrating necrotic morphology were characterized as effectively treated. In every RFA treated sample, the bone cortex along the periphery of the RFA treated region demonstrated an increased number of osteoblasts with new subcortical trabecular bone formation. The visible new bone formation seen only in the RFA treated groups averaged 4.04 ± 3.40 mm2 for the VX2-bearing femora and 5.78 ± 0.64 mm2 for the healthy femora (Fig. 5).
While H&E staining of specimens demonstrated some empty lacuna, a large number of healthy appearing osteocytes were visible post RFA treatment in both healthy and tumour-bearing groups. However, we noted that subsequent TUNEL staining demonstrated DNA fragmentation in many of these cells, indicating a RFA-driven treatment effect. We also observed intact osteocytes within the RFA treated region of 5 of 12 animals (Fig. 7). Increased TUNEL staining in the cortex was observed in 3 of 12 animals where the electrode applicator was fluoroscopically observed at the time of procedure to be touching the cortex.
Discussion
There is limited scientific literature concerning evaluation of RFA for bone tumours, in particular for structurally large bone lesions [13–15, 23, 24]. The available literature evaluates the efficacy of RFA in healthy bone as a model for metastases. In our study, RFA significantly reduced the number of viable tumour cells and suppressed growth of tumours when compared to non-RFA treated tumour controls (eight-fold reduction by tumour volume, p < 0.001). However, viable tumour cells were still observed in the RFA zone in one third of the RFA treated tumour bearing rabbits. Ablative effects were also observed on bone cells (osteocytes, osteoblasts, osteoclasts) and on periosteum and endosteum, within the treatment volumes as well as bone reactive changes in the skeletal periphery of the ablation zone.
Following RFA, a surprisingly large number of healthy appearing osteocytes were visible in both healthy and tumour-bearing cortical bone, although subsequent TUNEL staining demonstrated DNA fragmentation in many of these cells, indicating a RFA-driven treatment effect. This may be due to a temporal affect or the inherent tissue properties of bone, as described further below.
Wang et al. suggested that full RFA effects (in soft tissues) cannot be visualized using H&E in time points earlier than four weeks [25]. Similar to the current study, Yamamoto et al. found that RFA did induce bone cell death. They found at day 7 osteocytes shrank and periosteum disappeared in the centre of the cortical bone ablation area, with osteocytes no longer evident by day 30. This suggests that the changes seen in the osteocytes in the current study at day 14 post treatment may represent a treatment effect not fully realized from a temporal perspective. Similar to the increased number of osteoblasts with new subcortical trabecular bone formation found at the bone cortex along the periphery of the RFA treated region in our study, Yamamoto et al. also noted a proliferation of bone cells and new bone formation at the periphery of the RF ablation zone [26].
In bone, while Lundsgok reported instant necrosis of osteocytes after 30 s of exposure to temperatures of 50 °C [27], Tellotson et al. [15], experimenting with different monopolar RFA electrode applicators in healthy dog femora, found complete necrosis of bone marrow yet visible intact osteocytes and variable osteonecrosis of the cortical bone in the treatment zone. These findings were noted regardless of electrode applicator size evaluated or duration of heating. The conventional monopolar electrode applicators used in Tellotson’s work however, yielded only small zones of ablation (spherical, typically 1 cm in diameter). In an ex vivo RFA study of bovine bone, Rachbauer et al. observed that while the average temperature of the bone marrow 5 mm distant to the RF electrode applicator tip was 90.9 ± 26.0 °C, the average temperature recorded at the cortex 5 mm away was only 64.3 ± 13.7 °C and at 10 mm this thermal effect dropped to 41.7 ± 2.2 °C [13]. It may be possible that in vivo, the potential heat sinking effect of perfused blood vessels could lead to the generation of lower effective RFA temperatures, possibly leading to sparing of osteocytes.
The observed patchy necrosis of the osteocytes may also stem from the relative resistance of cortical bone and mineralized tissue to RFA effects (which may be ten to a hundred times greater when compared to solid organ tissues such as liver or bone marrow [13] ). This may explain the observed soft tissue necrosis adjacent to the cortical shell despite the seemingly healthy osteocytes within the cortical shell in our study. Thermal effects may be lessened by heat sink effects of the spinal fluid or nearby vasculature. An interesting observation of our study was the significant ablation effects of RFA on cortical bone in those femora that were fluoroscopically visualized during experimentation where the electrode applicator tip contacted the cortex. As such, intramedullary placement without cortical contact is desired to limit unnecessary cortical necrosis. It remains important therefore to optimize treatment parameters and electrode applicator positioning so as to target diseased tissue and preserve adjacent normal tissues.
Recent work in healthy rabbit femurs treated with RF ablation demonstrated similar high intensity rings on T2-weighted and STIR MR images at early time points as seen in healthy porcine vertebrae treated with BCRF and other reports in the literature [14, 26, 28, 29]. However, the high intensity rims decreased in size over time. These high intensity rims on T2-weighted in the outermost zones have been reported to correspond to granulation tissue, with hypo- and iso-intense areas in the inner zone corresponding to hemorrhagic congestion and coagulation necrosis [14]. No changes in the mean fracture loads of the RFA treated femora were found, suggesting that the heating did not impair bone stability up to 60 days post treatment [26].
Our observations of an increased number and size of the osteoclasts in the sham tumour bearing group (compared to the RFA treated tumour bearing group) are consistent with reported literature. Virmani et al. has highlighted the hypoxic nature of VX2 tumours [30] and Bozec et al. demonstrated that hypoxia induces an increase in the size of osteoclasts [5]. Since tumour osteolysis associated with metastasis is more aggressive at an early phase (when the tumour cells are establishing themselves and bone resorption proceeds rapidly, recruiting higher number of osteoclasts) [31], application of RFA for tumour control affecting osteoclastic activity may be desirable when treating during this earlier phase. In addition, an early planned stage of tumour ablation may result in a more homogenous lesion (since tumour necrosis and central tumour cavitation, a finding associated with advanced lesions, has not yet occurred).
Histological analysis confirmed a large region of RFA effect as visualized on MRI. Post-gadolinium imaging (Gd-SPGR and Gd-FIESTA) provided the best appreciation of RFA bone effects. From an imaging perspective, however, it remains challenging to distinguish between coagulated bone marrow and necrotized tumour within bone, even after the administration of the contrast agent.
Similar to our efforts, Proscheck et al. used metastatic nude rats (from human breast cancer cell line) to investigate efficacy of RFA in a preclinical tumour-bearing bone model. Although they demonstrated a successful RFA treatment, their average tumour area was 19 mm2, with tumour diameters ranging from 2–5 mm [23]. In contrast, the BCRF electrode applicator custom designed for intraosseous applications (by virtue of internal cooling, bipolarity and the active tip size) was able to ablate large zones including tumour tissue and bone (average 12.9 ± 5.5 cm3 volume of effect in this rabbit femur model). The size of the RF ablation zone achieved with a single treatment in this study incorporated both bone and surrounding thigh soft tissues. This observation was not unexpected as the study utilized an electrode applicator designed for human vertebral bone applied to the rabbit anatomy. The generated ablation zone is therefore large for rabbit femora and extended to the surrounding soft tissues. Yet the size of the lesions is encouraging from a translational perspective as they are volumetrically relevant in application to the human metastatic spine (Note: this electrode applicator has since been clinically approved for use in the treatment of human vertebral metastases). The safety of the BCRF electrode applicator has been previously demonstrated in a porcine vertebral study [28].
To date no consistent large animal vertebral tumour model exists, and as such, the VX2 lapine model was considered a good alternative that we tailored to best suit our study. Disadvantages of our model include its limited size (as described above) and a local injection protocol for emulating tumour metastasis. A VX2 tumour model of the spine is described in the rabbit, but is more of an epidural disease and neural model when compared to a bony vertebral metastatic model. As such a direct injection model of VX2 cells in the femur was utilized. Finally, while structural changes were evaluated histologically, no mechanical testing was performed to evaluate the impact of BCRF treatment on bone strength. Previous work has shown no change in the bone strength of rabbit femora post RFA treatment up to 2 months (25).
Conclusions
Structurally large VX2 tumour volumes within bone were successfully ablated, stimulating new bone formation in the periphery of the targeted treatment zone. The ablation zone was best distinguished from remaining tumour and healthy tissues using contrast enhanced MR imaging, although histologically, viable appearing osteocytes and tumour cells were observed in some treated regions. The existence of select sporadic viable tumour cells within the RF ablation zone in a subset of animals motivates the desire for further improvements in zonal ablation.
Abbreviations
- RFA:
-
Radiofrequency ablation
- BCRF:
-
Bipolar cooled radiofrequency ablation
- NZW:
-
New Zealand white
- Oc:
-
Osteoclasts
- TRAP:
-
Tartrate-resistant acid phosphatase
- H&E:
-
Hematoxylin and eosin
- TUNEL:
-
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- SPGR:
-
SPoiled Gradient Recalled
- FIESTA:
-
Fast imaging employing steady state acquisition
- Gd:
-
Gadolinium
References
Coleman RE (2001) Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 27(3):165–176
Albisinni U, Rimondi E, Malaguti M et al (2005) Radiofrequency thermal ablation of non spinal osteoid osteoma: remarks on method. Radiol Med 109(5–6):555
Cantwell C, O’Byrne J, Eustace S (2006) Radiofrequency ablation of osteoid osteoma with cooled probes and impedance-control energy delivery. AJR Am J Roentgenol 186(5 Suppl):S244
Cristante A, Barros Filho T, Oliveira R et al (2007) Treatment of osteoid osteoma in the vertebral body of the lumbar spine by radiofrequency ablation. Clinics 62(6):791
Bozec A, Bakiri L, Hoebertz A et al (2008) Osteoclast size is controlled by Fra-2 through LIF/LIF-receptor signalling and hypoxia. Nature 454(7201):221
Callstrom M, Charboneau J, Goetz M et al (2002) Painful metastases involving bone: feasibility of percutaneous CT- and US-guided radio-frequency ablation. Radiology 224(1):87
Kashima M, Yamakado K, Takaki H et al (2010) Radiofrequency ablation for the treatment of bone metastases from hepatocellular carcinoma. AJR Am J Roentgenol 194(2):536
Callstrom MR, York JD, Gaba RC et al (2009) Research reporting standards for image-guided ablation of bone and soft tissue tumors. J Vasc Interv Radiol 20(12):1527–1540
Solbiati L, Ahmed M, Cova L et al (2010) Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology 265(3):958
Al-Omari M, Ata K, Al-Muqbel K et al (2012) Radiofrequency ablation of osteoid osteoma using tissue impedance as a parameter of osteonecrosis. J Med Imaging Radiat Oncol 56(4):384
Langberg J, Lee M, Chin M et al (1990) Radiofrequency catheter ablation: the effect of electrode size on lesion volume in vivo. Pacing Clin Electrophysiol 13(10):1242–1248
Liu Z, Lobo S, Humphries S et al (2005) Radiofrequency tumor ablation: insight into improved efficacy using computer modeling. AJR Am J Roentgenol 184(4):1347
Rachbauer F, Mangat J, Bodner G et al (2003) Heat distribution and heat transport in bone during radiofrequency catheter ablation. Arch Orthop Trauma Surg 123(2–3):86
Lee J, Choi S, Park H et al (2005) Radiofrequency thermal ablation in canine femur: evaluation of coagulation necrosis reproducibility and MRI-histopathologic correlation. AJR Am J Roentgenol 185(3):661
Tillotson C, Rosenberg A, Rosenthal D (1989) Controlled thermal injury of bone. report of a percutaneous technique using radiofrequency electrode and generator. Invest Radiol 24(11):888
Galasko C (1981) Bone metastases studied in experimental animals. Clin Orthop Relat Res 155:269
Ikenaga M, Ohura K, Yamamuro T et al (1993) Localized hyperthermic treatment of experimental bone tumors with ferromagnetic ceramics. J Orthop Res 11(6):849–855
Akagi M, Tsuboyama T, Ikenaga M et al (1997) Anti-tumour effects of localized hyperthermia on an experimental bone tumour using an intramedullary nail. Int J Hyperthermia 13(4):387–400
Kusaka M, Takegami K, Sudo A et al (2007) Effect of hyperthermia by magnetite cement on tumor-induced bone destruction. J Orthop Sci 7(3):354–357
Choi J, Kang E, Kim H et al (2008) Evolution of VX2 carcinoma in rabbit tibia: magnetic resonance imaging with pathologic correlation. Clin Imaging 32(2):128–135
Callis G, Sterchi D (1998) Decalcification of bone: literature review and practical study of various decalcifying agents. methods, and their effects on bone histology. J Histotechnol 21(1):49
Vernau KM, Osofsky A, LeCouteur RA (2007) The neurological examination and lesion localization in the companion rabbit (Oryctolagus cuniculus). Vet Clin North Am 10(3):731
Proschek D, Mack MG, Kurth AA et al (2008) Radiofrequency ablation of experimental bone metastases in nude rats. Anticancer Res 28(2A):879–885
Sciubba DM, Burdette EC, Cheng JJ et al (2010) Percutaneous computed tomography fluoroscopy-guided conformal ultrasonic ablation of vertebral tumors in a rabbit tumor model. Laboratory investigation. J Neurosurg Spine 13(6):773–779
Wang Q, Huang J, Ma K et al (2012) Evaluation of ghost cell survival in the area of radiofrequency ablation. PLoS One 7(12):e53158
Yamamoto S, Kaminou T, Ono Y et al (2014) Thermal influence of radiofrequency ablation for bone: an experimental study in normal rabbit bone. Skeletal Radiol 43(4):459–465
Lundskog J (1972) Heat and bone tissue. An experimental investigation of the thermal properties of bone and threshold levels for thermal injury. Scand J Plast Reconstr Surg 9:1
Pezeshki PS, Woo J, Akens MK et al (2014) Evaluation of a bipolar-cooled radiofrequency device for ablation of bone metastases: preclinical assessment in porcine vertebrae. Spine J 14(2):361–370
Nour S, Aschoff A, Mitchell I et al (2002) MR imaging-guided radio-frequency thermal ablation of the lumbar vertebrae in porcine models. Radiology 224(2):452
Virmani S, Rhee T, Ryu R et al (2008) Comparison of hypoxia-inducible factor-1alpha expression before and after transcatheter arterial embolization in rabbit VX2 liver tumors. J Vasc Interv Radiol 19(10):1483
Singh G (2004) Section 4. In: Bone metastasis and molecular mechanisms: pathophysiology. Springer, New York
Acknowledgments
This research was supported by a peer-reviewed grant from the Ontario Centers of Excellence (OCE) with a matching in kind contribution from Baylis Medical Inc., Mississauga, Ontario. We thank Dr. Rita Kandel for her assistance with histological interpretation, Ms. Sara Moore and Ms. Carrie Purcell for their assistance with the animal studies and Mr. Firas Moosvi for his assistance with the MR imaging.
Conflict of Interest
Co-author JW is an employee of Baylis Medical Inc. This author was not involved in the image/data analysis or animal evaluations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pezeshki, P.S., Akens, M.K., Gofeld, M. et al. Bone targeted bipolar cooled radiofrequency ablation in a VX-2 rabbit femoral carcinoma model. Clin Exp Metastasis 32, 279–288 (2015). https://doi.org/10.1007/s10585-015-9703-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-015-9703-8