Abstract
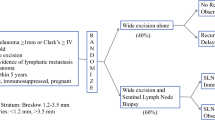
This short review offers an update on the first and second Multicenter Selective Lymphadenectomy Trials (MSLT-I and MSLT-II) for patients with melanoma, and briefly traces the development of intraoperative lymphatic mapping and sentinel node biopsy.








Similar content being viewed by others
References
Balch CM, Soong SJ, Gershenwald JE et al (2001) Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol 19:3622–3634
Morton DL, Hoon DS, Cochran AJ et al. (2003) Lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: therapeutic utility and implications of nodal microanatomy and molecular staging for improving the accuracy of detection of nodal micrometastases. Ann Surg 238:538–49 (discussion 49–50)
Holmes EC, Moseley HS, Morton DL, Clark W, Robinson D, Urist MM (1977) A rational approach to the surgical management of melanoma. Ann Surg 186:481–490
Morton DL, Cochran AJ, Thompson JF et al. (2005) Sentinel node biopsy for early-stage melanoma: accuracy and morbidity in MSLT-I, an international multicenter trial. Ann Surg 242:302–11 (discussion 11–3)
Balch CM, Soong S, Ross MI et al (2000) Long-term results of a multi-institutional randomized trial comparing prognostic factors and surgical results for intermediate thickness melanomas (1.0 to 4.0 mm). Intergroup Melanoma Surgical Trial. Ann Surg Oncol 7:87–97
Robinson DS, Sample WF, Fee HJ, Holmes C, Morton DL (1977) Regional lymphatic drainage in primary malignant melanoma of the trunk determined by colloidal gold scanning. Surg Forum 28:147–148
Wong JH, Cagle LA, Morton DL (1991) Lymphatic drainage of skin to a sentinel lymph node in a feline model. Ann Surg 214:637–641
Morton DL, Wen DR, Wong JH et al (1992) Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg 127:392–399
Morton DL, Thompson JF, Essner R et al. (1999) Validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: a multicenter trial. Multicenter Selective Lymphadenectomy Trial Group. Ann Surg 230:453–63 (discussion 63–5)
Morton DL, Cochran AJ, Thompson JF (2007) Sentinel node biopsy in melanoma (letter). N Engl J Med 356:419–420
Balch CM, Soong SJ, Murad TM, Ingalls AL, Maddox WA (1981) A multifactorial analysis of melanoma: III. Prognostic factors in melanoma patients with lymph node metastases (stage II). Ann Surg 193:377–388
Balch CM, Buzaid AC, Soong SJ et al (2001) Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 19:3635–3648
Morton DL, Thompson JF, Cochran AJ et al (2006) Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med 355:1307–1317
Morton DL, Cochran AJ, Thompson JF (2007) Authors’ response to a letter to the editor re: sentinel node biopsy for early-stage melanoma. Ann Surg 245:828–829
Faries MB, Thompson JF, Cochran A et al (2010) The impact on morbidity and length of stay of early versus delayed complete lymphadenectomy in melanoma: results of the Multicenter Selective Lymphadenectomy Trial (I). Ann Surg Oncol 17:3324–3329
Garreau JR, Faries M, Ye X (2009) Mood state and melanoma outcome in the Multicenter Selective Lymphadenectomy Trial. J Clin Oncol 27:Abstract 9603
Cochran AJ, Wen DR, Huang RR, Wang HJ, Elashoff R, Morton DL (2004) Prediction of metastatic melanoma in nonsentinel nodes and clinical outcome based on the primary melanoma and the sentinel node. Mod Pathol 17:747–755
Acknowledgments
Thanks to all members of the MSLT Study Group and to all patients in the United States, Europe and Australia whose participation in these important trials has ensured their timely completion and successful outcome. Supported by a grant (CA 29605) from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Cancer Institute or the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article was conducted on behalf of the MSLT Study Group.
The details of the Multicenter Selective Lymphadenectomy Trial participants are given in Appendix.
Appendix
Appendix
The Multicenter Selective Lymphadenectomy Trial participants are as follows: Independent Data and Safety Monitoring Committee: John Kirkwood, MD., Chairman; John Daly, MD.; Michael Kutner, PhD; Martin Mihm, MD.; Gary Smith, MD.; Marshal Urist, MD.; and Patient Advocate, Norman Beegun, Esq. Trial sites (Principal investigators): Sydney Melanoma Unit, Sydney, Australia (John F. Thompson, MD); Istituto Nazionale dei Tumori de Napoli, Naples, Italy (Nicola Mozzillo, MD); Netherlands Cancer Institute, Amsterdam, The Netherlands (Omgo E. Nieweg, MD, PhD); New York University, New York, New York (Daniel F. Roses, MD); University Medical Center Groningen and University of Groningen, Groningen, The Netherlands (Harald J. Hoekstra MD, PhD); Millard Fillmore Hospital, Buffalo, New York (Constantine P. Karakousis, MD, PhD); H. Lee Moffitt Cancer Center, Tampa, Florida (Vernon Sondak, MD, and Douglas S. Reintgen, MD); University of California, San Francisco, Mt. Zion Medical Center (Stanley P.L. Leong, MD); Royal Adelaide Hospital, Adelaide, Australia (Brendon J. Coventry, BM, BS, PhD); Roswell Park Cancer Institute, Buffalo, New York (William Kraybill, MD); Princess Alexandra Hospital, Brisbane, Australia (Mark Smithers, MB, BS); Henry Ford Hospital, Detroit, Michigan (S. David Nathanson, MD); University of Texas Southwestern Medical Center at Dallas, Dallas, Texas (James F. Huth, MD); University of Hawaii at Manoa, Honolulu, Hawaii (Jan H. Wong, MD); University of Pennsylvania (Douglas L. Fraker, MD); Tom Baker Cancer Centre, Calgary, Alberta, Canada (Walley Temple, MD); Nuernberg Hospital, Germany (Eberhard Paul, MD); John Wayne Cancer Institute, Santa Monica, California (Donald L. Morton, MD).
Rights and permissions
About this article
Cite this article
Morton, D.L. Overview and update of the phase III Multicenter Selective Lymphadenectomy Trials (MSLT-I and MSLT-II) in melanoma. Clin Exp Metastasis 29, 699–706 (2012). https://doi.org/10.1007/s10585-012-9503-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-012-9503-3