Abstract
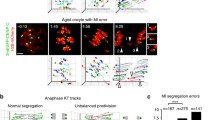
Mammalian female gametes frequently suffer from numerical chromosomal aberrations, the main cause of miscarriages and severe developmental defects. The underlying mechanisms responsible for the development of aneuploidy in oocytes are still not completely understood and remain a subject of extensive research. From studies focused on prevalence of aneuploidy in mouse oocytes, it has become obvious that reported rates of aneuploidy are strongly dependent on the method used for chromosome counting. In addition, it seems likely that differences between mouse strains could influence the frequency of aneuploidy as well; however, up till now, such a comparison has not been available. Therefore, in our study, we measured the levels of aneuploidy which has resulted from missegregation in meiosis I, in oocytes of three commonly used mouse strains—CD-1, C3H/HeJ, and C57BL/6. Our results revealed that, although the overall chromosomal numerical aberration rates were similar in all three strains, a different number of oocytes in each strain contained prematurely segregated sister chromatids (PSSC). This indicates that a predisposition for this type of chromosome segregation error in oocyte meiosis I is dependent on genetic background.




Similar content being viewed by others
Abbreviations
- CGH:
-
Comparative genomic hybridization
- CSF:
-
Cytostatic factor
- FISH:
-
Fluorescence in situ hybridization
- GV:
-
Germinal vesicle
- GVBD:
-
Germinal vesicle breakdown
- PB:
-
Polar body
- PSSC:
-
Prematurely segregated sister chromatids/precociously separated sister chromatids
- SAC:
-
Spindle assembly checkpoint
References
Aldinger KA, Sokoloff G, Rosenberg DM, Palmer AA, Millen KJ (2009) Genetic variation and population substructure in outbred CD-1 mice: implications for genome-wide association studies. PLoS One 4:e4729
Angell R (1997) First-meiotic-division nondisjunction in human oocytes. Am J Hum Genet 61:23–32
Cheng PP, Xia JJ, Wang HL et al (2011) Islet transplantation reverses the effects of maternal diabetes on mouse oocytes. Reproduction 141:417–424
Chiang T, Duncan FE, Schindler K, Schultz RM, Lampson MA (2010) Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr Biol 20:1522–1528
Colls P, Escudero T, Fischer J et al (2012) Validation of array comparative genome hybridization for diagnosis of translocations in preimplantation human embryos. Reprod BioMed Online 24:621–629
Duncan FE, Chiang T, Schultz RM, Lampson MA (2009) Evidence that a defective spindle assembly checkpoint is not the primary cause of maternal age-associated aneuploidy in mouse eggs. Biol Reprod 81:768–776
Eichenlaub-Ritter U (1998) Genetics of oocyte ageing. Maturitas 30:143–169
Eichenlaub-Ritter U (2012) Oocyte ageing and its cellular basis. Int J Dev Biol 56:841–852
Fragouli E, Alfarawati S, Goodall NN, Sanchez-Garcia JF, Colls P, Wells D (2011) The cytogenetics of polar bodies: insights into female meiosis and the diagnosis of aneuploidy. Mol Hum Reprod 17:286–295
Fragouli E, Alfarawati S, Spath K et al (2013) The origin and impact of embryonic aneuploidy. Hum Genet 132:1001–1013
Gianaroli L, Magli MC, Cavallini G et al (2010) Predicting aneuploidy in human oocytes: key factors which affect the meiotic process. Hum Reprod 25:2374–2386
Golbus MS (1981) The influence of strain, maternal age, and method of maturation on mouse oocyte aneuploidy. Cytogenet Cell Genet 31:84–90
Gregan J, Polakova S, Zhang L, Tolic-Norrelykke IM, Cimini D (2011) Merotelic kinetochore attachment: causes and effects. Trends Cell Biol 21:374–381
Gutierrez-Mateo C, Colls P, Sanchez-Garcia J et al (2011) Validation of microarray comparative genomic hybridization for comprehensive chromosome analysis of embryos. Fertil Steril 95:953–958
Hansmann I (1974) Chromosome aberrations in metaphase II-oocytes. Stage sensitivity in the mouse oogenesis to amethopterin and cyclophosphamide. Mutat Res 22:175–191
Hassold T, Chiu D (1985) Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum Genet 70:11–17
Hassold T, Hunt P (2001) To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet 2:280–291
Hassold T, Hunt P (2009) Maternal age and chromosomally abnormal pregnancies: what we know and what we wish we knew. Curr Opin Pediatr 21:703–708
Hassold TJ, Jacobs PA (1984) Trisomy in man. Annu Rev Genet 18:69–1897
Hassold T, Hall H, Hunt P (2007) The origin of human aneuploidy: where we have been, where we are going. Hum Mol Genet 16(2):203–208
Hornak M, Jeseta M, Musilova P et al (2011) Frequency of aneuploidy related to age in porcine oocytes. PLoS One 6:e18892
Howe K, FitzHarris G (2013) Recent insights into spindle function in mammalian oocytes and early embryos. Biol Reprod 89:71
Hunt P, Hassold T (2010) Female meiosis: coming unglued with age. Curr Biol 20:699–702
Jacobs PA (1992) The chromosome complement of human gametes. Oxf Rev Reprod Biol 14:47–72
Jamieson ME, Coutts JR, Connor JM (1994) The chromosome constitution of human preimplantation embryos fertilized in vitro. Hum Reprod 9:709–715
Jones KT, Lane SI (2013) Molecular causes of aneuploidy in mammalian eggs. Development 140:3719–3730
Kuliev A, Cieslak J, Verlinsky Y (2005) Frequency and distribution of chromosome abnormalities in human oocytes. Cytogenet Genome Res 111:193–198
Kuliev A, Zlatopolsky Z, Kirillova I, Spivakova J, Cieslak Janzen J (2011) Meiosis errors in over 20,000 oocytes studied in the practice of preimplantation aneuploidy testing. Reprod BioMed Online 22:2–8
Lechniak D, Switonski M (1998) Aneuploidy in bovine oocytes matured in vitro. Chromosom Res 6:504–506
Lister LM, Kouznetsova A, Hyslop LA et al (2010) Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr Biol 20:1511–1521
Madgwick S, Jones KT (2007) How eggs arrest at metaphase II: MPF stabilisation plus APC/C inhibition equals cytostatic factor. Cell Div 2:4
Mailhes JB, Hilliard C, Lowery M, London SN (2002) MG-132, an inhibitor of proteasomes and calpains, induced inhibition of oocyte maturation and aneuploidy in mouse oocytes. Cell Chromosome 1:2
Martin RH, Ko E, Rademaker A (1991) Distribution of aneuploidy in human gametes: comparison between human sperm and oocytes. Am J Med Genet 39:321–331
Merriman JA, Jennings PC, McLaughlin EA, Jones KT (2011) Effect of aging on superovulation efficiency, aneuploidy rates, and sister chromatid cohesion in mice aged up to 15 months. Biol Reprod 86:49
Merriman JA, Lane SI, Holt JE et al (2013) Reduced chromosome cohesion measured by interkinetochore distance is associated with aneuploidy even in oocytes from young mice. Biol Reprod 88:31
Munne S, Chen S, Colls P et al (2007) Maternal age, morphology, development and chromosome abnormalities in over 6000 cleavage-stage embryos. Reprod BioMed Online 14:628–634
Nagaoka SI, Hassold TJ, Hunt PA (2012) Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet 13:493–504
O’Connell CB, Loncarek J, Hergert P, Kourtidis A, Conklin DS, Khodjakov A (2008) The spindle assembly checkpoint is satisfied in the absence of interkinetochore tension during mitosis with unreplicated genomes. J Cell Biol 183:29–36
Pan H, Ma P, Zhu W, Schultz RM (2008) Age-associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev Biol 316:397–407
Polanski Z, Ledan E, Brunet S et al (1998) Cyclin synthesis controls the progression of meiotic maturation in mouse oocytes. Development 125:4989–4997
Revenkova E, Herrmann K, Adelfalk C, Jessberger R (2010) Oocyte cohesin expression restricted to predictyate stages provides full fertility and prevents aneuploidy. Curr Biol 20:1529–1533
Rohrborn G, Hansmann I (1974) Oral contraceptives and chromosome segregation in oocytes of mice. Mutat Res 26:535–544
Rosenbusch B (2004) The incidence of aneuploidy in human oocytes assessed by conventional cytogenetic analysis. Hereditas 141:97–105
Rosenbusch B (2006) The contradictory information on the distribution of non-disjunction and pre-division in female gametes. Hum Reprod 21:2739–2742
Rosenbusch B, Glaeser B, Schneider M (2001) Aneuploidy in human oocytes: nondisjunction or predivision? Cytogenet Cell Genet 94:241–243
Sears DD, Hegemann JH, Hieter P (1992) Meiotic recombination and segregation of human-derived artificial chromosomes in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 89:5296–5300
Sebestova J, Danylevska A, Novakova L, Kubelka M, Anger M (2012) Lack of response to unaligned chromosomes in mammalian female gametes. Cell Cycle 11:3011–3018
Shomper M, Lappa C, FitzHarris G (2014) Kinetochore microtubule establishment is defective in oocytes from aged mice. Cell Cycle 13:1171–1179
Tachibana-Konwalski K, Godwin J, van der Weyden L et al (2010) Rec8-containing cohesin maintains bivalents without turnover during the growing phase of mouse oocytes. Genes Dev 24:2505–2516
Vialard F, Petit C, Bergere M et al (2006) Evidence of a high proportion of premature unbalanced separation of sister chromatids in the first polar bodies of women of advanced age. Hum Reprod 21: 1172–1178
Yun Y, Lane SI, Jones KT (2014a) Premature dyad separation in meiosis II is the major segregation error with maternal age in mouse oocytes. Development 141:199–208
Yun Y, Holt JE, Lane SI, McLaughlin EA, Merriman JA, Jones KT (2014b) Reduced ability to recover from spindle disruption and loss of kinetochore spindle assembly checkpoint proteins in oocytes from aged mice. Cell Cycle 13
Zackowski JL, Martin-Deleon PA (1988) Second meiotic nondisjunction is not increased in postovulatory aged murine oocytes fertilized in vitro. In vitro Cell Dev Biol 24:133–137
Zuccotti M, Boiani M, Garagna S, Redi CA (1998) Analysis of aneuploidy rate in antral and ovulated mouse oocytes during female aging. Mol Reprod Dev 50:305–312
Acknowledgments
We are grateful to Jiri Rubes for helpful discussion and useful comments. This work was supported by Czech science foundation project P502/12/2201 and by MEYS projects ED1.1.00/02.0068—CEITEC, CZ.1.07/2.3.00/20.0213—CeDiLa, and LH 13072—Kontakt II.
Ethical standards
The authors declare that all experiments performed in this study comply with the current laws of the Czech Republic. All institutional and national guidelines for the care and use of laboratory animals were followed.
Conflict of interest
The authors (A.D., K.K., T.A., and M.A.) declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Fengtang Yang
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure 1
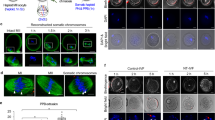
Resolution of the superimposed or closely positioned kinetochores by 3D reconstruction. The left image shows a chromosome with a pair of superimposed kinetochores. The middle image presents the same chromosome rotated 90 degrees along the z-axis. The image on the right only shows the kinetochore channel of the same chromosome. Chromosomes (in red) stained with DAPI, kinetochores (in green) labeled by CREST antiserum. Scale bar represents 1 μm. (GIF 82 kb)
Rights and permissions
About this article
Cite this article
Danylevska, A., Kovacovicova, K., Awadova, T. et al. The frequency of precocious segregation of sister chromatids in mouse female meiosis I is affected by genetic background. Chromosome Res 22, 365–373 (2014). https://doi.org/10.1007/s10577-014-9428-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-014-9428-6