Abstract
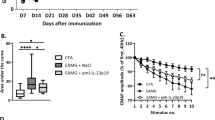
Myasthenia gravis (MG) is an autoantibody-mediated autoimmune disease characterized by skeletal muscle weakness exacerbated with exercise. There is a need for novel drugs effective in refractory MG. We aimed to test the potential of teriflunomide, an immunomodulatory drug currently used in rheumatoid arthritis and multiple sclerosis treatment, in a murine experimental autoimmune myasthenia gravis (EAMG) model. EAMG was induced by immunizations with recombinant acetylcholine receptor (AChR). Teriflunomide treatment (10 mg/kg/day, intraperitoneal) was initiated to one group of mice (n = 21) following the third immunization and continued for 5 weeks. The disease control group (n = 19) did not receive medication. Naïve mice (n = 10) received only mock immunization. In addition to the clinical scorings, the numbers of B cells and T cells, and cytokine profiles of T cells were examined by flow cytometry. Anti-AChR-specific antibodies in the peripheral blood serum were quantified by ELISA. Teriflunomide significantly reduced clinical disease scores and the absolute numbers of CD4+ T cells and some of their cytokine-producing subgroups (IFN-γ, IL 2, IL22, IL-17A, GM-CSF) in the spleen and the lymph nodes. The thymic CD4+ T cells were also significantly reduced. Teriflunomide mostly spared CD8+ T cells’ numbers and cytokine production, while reducing CD138+CD19+lambda+ plasma B cells’ absolute numbers and CD138 mean fluorescent intensities, probably decreasing the number of IgG secreting more mature plasma cells. It also led to some selective changes in the measurements of anti-AChR-specific antibodies in the serum. Our results showed that teriflunomide may be beneficial in the treatment of MG in humans.
Graphical Abstract












Similar content being viewed by others
Data Availability
Enquiries about data availability should be directed to the authors.
References
Amaldi I, Reith W, Berte C, Mach B (1989) Induction of HLA class II genes by IFN-gamma is transcriptional and requires a trans-acting protein. J Immunol 142:999–1004
Bar-Or A, Pachner A, Menguy-Vacheron F, Kaplan J, Wiendl H (2014) Teriflunomide and its mechanism of action in multiple sclerosis. Drugs 74:659–674. https://doi.org/10.1007/s40265-014-0212-x
Bortnick A, Allman D (2013) What is and what should always have been: long-lived plasma cells induced by T cell-independent antigens. J Immunol 190:5913–5918. https://doi.org/10.4049/jimmunol.1300161
Carr AS, Cardwell CR, McCarron PO, McConville J (2010) A systematic review of population based epidemiological studies in Myasthenia Gravis. BMC Neurol 10:1–9. https://doi.org/10.1186/1471-2377-10-46
Claussen MC, Korn T (2012) Immune mechanisms of new therapeutic strategies in MS- Teriflunomide. Clin Immunol 142:49–56. https://doi.org/10.1016/j.clim.2011.02.011
Committee for Medicinal Products for Human Use (CHMP) (2013) Assessment report AUBAGIO, International mon-proprietary name: TERIFLUNOMIDE Procedure No. EMEA/H/C/002514/0000. https://www.ema.europa.eu/documents/assessment-report/aubagio-epar-public-assessment-report_en.pdf. Accessed 27 March 2022
Confavreux C, O’Connor P, Comi G, Freedman MS, Miller AE, Olsson TP, Wolinsky JS, Bagulho T, Delhay JL, Dukovic D, Truffinet P, Kappos L, Group T.T. (2014) Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 13:247–256. https://doi.org/10.1016/S1474-4422(13)70308-9
Dhillon S (2018) A review in generalized myasthenia gravis. Drugs 78(5):607. https://doi.org/10.1007/s40265-018-0889-3
Ey PL, Prowse SJ, Jenkin CR (1979) Complement-fixing IgG1 constitutes a new subclass of mouse IgG. Nature 281:492–493. https://doi.org/10.1038/281492a0
Fichtner ML, Jiang R, Bourke A, Nowak RJ, O’Connor KC (2020) Autoimmune pathology in myasthenia gravis disease subtypes is governed by divergent mechanisms of immunopathology. Front Immunol 11:e00776. https://doi.org/10.3389/fimmu.2020.00776
Filosso PL, Galassi C, Ruffini E, Margaritora S, Bertolaccini L, Casadio C, Anile M, Venuta F (2013) Thymoma and the increased risk of developing extrathymic malignancies: a multicentre study. Eur J Cardiothorac Surg 44:219–224. https://doi.org/10.1093/ejcts/ezs663 (discussion 224)
Gandoglia I, Ivaldi F, Laroni A, Benvenuto F, Solaro C, Mancardi G, Kerlero de Rosbo N, Uccelli A (2017) Teriflunomide treatment reduces B cells in patients with MS. Neurol Neuroimmunol Neuroinflamm 4:e403. https://doi.org/10.1212/NXI.0000000000000403
Higuchi O, Hamuro J, Motomura M, Yamanashi Y (2011) Autoantibodies to low-density lipoprotein receptor-related protein 4 in myasthenia gravis. Ann Neurol 69:418–422. https://doi.org/10.1002/ana.22312
Hoch W, McConville J, Helms S, Newsom-Davis J, Melms A, Vincent A (2001) Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med 7:365–368. https://doi.org/10.1038/85520
Howard JF Jr (2018) Myasthenia gravis: the role of complement at the neuromuscular junction. Ann N Y Acad Sci 1412:113–128. https://doi.org/10.1111/nyas.13522
Khodadadi L, Cheng QY, Radbruch A, Hiepe F (2019) The maintenance of memory plasma cells. Front Immunol 10:721. https://doi.org/10.3389/fimmu.2019.00721
Lefvert AK, Cuenoud S, Fulpius BW (1981) Binding-properties and subclass distribution of anti-acetylcholine Receptor antibodies in myasthenia-gravis. J Neuroimmunol 1:125–135. https://doi.org/10.1016/0165-5728(81)90015-1
Li L, Liu JC, Delohery T, Zhang DH, Arendt C, Jones C (2013) The effects of teriflunomide on lymphocyte subpopulations in human peripheral blood mononuclear cells in vitro. J Neuroimmunol 265:82–90. https://doi.org/10.1016/j.jneuroim.2013.10.003
Loffler M, Klein A, Hayek-Ouassini M, Knecht W, Konrad L (2004) Dihydroorotate dehydrogenase mRNA and protein expression analysis in normal and drug-resistant cells. Nucleosides Nucleotides Nucleic Acids 23:1281–1285. https://doi.org/10.1081/NCN-200027547
Mantegazza R, Cordiglieri C, Consonni A, Baggi F (2016) Animal models of myasthenia gravis: utility and limitations. Int J Gen Med 9:53–64. https://doi.org/10.2147/IJGM.S88552
McConville J, Farrugia ME, Beeson D, Kishore U, Metcalfe R, Newsom-Davis J, Vincent A (2004) Detection and characterization of MuSK antibodies in seronegative myasthenia gravis. Ann Neurol 55:580–584. https://doi.org/10.1002/ana.20061
Moens L, Tangye SG (2014) Cytokine-mediated regulation of plasma cell generation: IL-21 takes center stage. Front Immunol 5:65. https://doi.org/10.3389/fimmu.2014.00065
Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM (2015) The generation of antibody-secreting plasma cells. Nat Rev Immunol 15:160–171. https://doi.org/10.1038/nri3795
O’Connor P, Wolinsky JS, Confavreux C, Comi G, Kappos L, Olsson TP, Benzerdjeb H, Truffinet P, Wang L, Miller A, Freedman MS (2011) Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 365:1293–1303. https://doi.org/10.1056/NEJMoa1014656
Posevitz V, Chudyka D, Kurth F, Wiendl H (2012) Teriflunomide suppresses antigen induced T-cell expansion in a TCR avidity dependent fashion. Mult Scler J 18:519–519. https://doi.org/10.1126/scitranslmed.aao5563
Ringshausen I, Oelsner M, Bogner C, Peschel C, Decker T (2008) The immunomodulatory drug Leflunomide inhibits cell cycle progression of B-CLL cells. Leukemia 22:635–638. https://doi.org/10.1038/sj.leu.2404922
Rodgaard A, Nielsen FC, Djurup R, Somnier F, Gammeltoft S (1987) Acetylcholine-Receptor Antibody in Myasthenia-Gravis—Predominance of Igg Subclass-1 and Subclass-3. Clin Exp Immunol 67:82–88
Ruckemann K, Fairbanks LD, Carrey EA, Hawrylowicz CM, Richards DF, Kirschbaum B, Simmonds HA (1998) Leflunomide inhibits pyrimidine de novo synthesis in mitogen-stimulated T-lymphocytes from healthy humans. J Biol Chem 273:21682–21691. https://doi.org/10.1074/jbc.273.34.21682
Shigemoto K, Takashima R, Motohashi N, Mori S (2015) Animal models of myasthenia gravis. Nihon Rinsho 73(Suppl 7):97–104. https://doi.org/10.1006/clim.1999.4807
Siemasko K, Chong AS-F, Williams JW, Bremer EG, Finnegan A (1996) Regulation of B cell function by the immunosupressive agent leflunomide. Transplantation 61:635–642. https://doi.org/10.1097/00007890-199602270-00020
Siemasko K, Chong AS-F, Jack HM, Gong H, Williams JW, Finnegan A (1998) Inhibition oj JAK3 and STAT6 tyrosine phosphorylation by the immunosupressive drug leflunomide leads to a block in IgG1 production. J Immunol 160:1581–1588
Silvestri NJ, Wolfe GI (2014) Treatment-refractory myasthenia gravis. J Clin Neuromuscul Dis 15:167–178. https://doi.org/10.1097/CND.0000000000000034
Sonkar KK, Bhoi SK, Dubey D, Kalita J, Misra UK (2017) Direct and indirect cost of myasthenia gravis: a prospective study from a tertiary care teaching hospital in India. J Clin Neurosci 38:114–117. https://doi.org/10.1016/j.jocn.2016.11.003
Stathopoulos P, Kumar A, Nowak RJ, O’Connor KC (2017) Autoantibody-producing plasmablasts after B cell depletion identified in muscle-specific kinase myasthenia gravis. JCI Insight 2:94263. https://doi.org/10.1172/jci.insight.94263
Tilly G, Cadoux M, Garcia A, Morille J, Wiertlewski S, Pecqueur C, Brouard S, Laplaud D, Degauque N (2021) Teriflunomide treatment of multiple sclerosis selectively modulates CD8 memory T cells. Front Immunol 12:730342. https://doi.org/10.3389/fimmu.2021.730342
Tuzun E, Berrih-Aknin S, Brenner T, Kusner LL, Le Panse R, Yang H, Tzartos S, Christadoss P (2015) Guidelines for standard preclinical experiments in the mouse model of myasthenia gravis induced by acetylcholine receptor immunization. Exp Neurol 270:11–17. https://doi.org/10.1016/j.expneurol.2015.02.009
Ulusoy C, Cavus F, Yilmaz V, Tuzun E (2017) Immunization with recombinantly expressed LRP4 induces experimental autoimmune myasthenia gravis in C57BL/6 mice. Immunol Invest 46:490–499. https://doi.org/10.1080/08820139.2017.1299754
Uzawa A, Kuwabara S, Suzuki S, Imai T, Murai H, Ozawa Y, Yasuda M, Nagane Y, Utsugisawa K (2021) Roles ofcytokines and T cells in the pathogenesis of myasthenia gravis. Clin Exp Immunol 203(3):366–374. https://doi.org/10.1111/cei.13546
Vincent A (2002) Unravelling the pathogenesis of myasthenia gravis. Nat Rev Immunol 2:797–804
Wang Z, Yan YP (2017) Immunopathogenesis in Myasthenia Gravis and Neuromyelitis Optica. Front Immunol 8:1785. https://doi.org/10.1038/nri916
Wiendl H, Seze JD, Cross AH, Winthrop K, Gupta AD, Kerloeguen C, Pingili R, Sullivam R, Ramanathan K, Kappos L (2020) Serum immunoglobulin levels and infections in relapsing multiple sclerosis. https://www.charcot-ms.org/28th-annual-meeting-digital/poster-presentations/clinical/heinz-wiendl-33
Yilmaz V, Ulusoy C, Hajtovic S, Turkoglu R, Kurtuncu M, Tzartos J, Lazaridis K, Tuzun E (2021) Effects of Teriflunomide on B cell subsets in MuSK-induced experimental autoimmune myasthenia gravis and multiple sclerosis. Immunol Invest 50:671–684. https://doi.org/10.1080/08820139.2020.1785491
Zhang GX, Xiao BG, Bakhiet M, van der Meide P, Wigzell H, Link H, Olsson T (1996) Both CD4(+) and CD8(+) T cells are essential to induce experimental autoimmune myasthenia gravis. J Exp Med 184:349–356. https://doi.org/10.1084/jem.184.2.349
Zisimopoulou P, Evangelakou P, Tzartos J, Lazaridis K, Zouvelou V, Mantegazza R, Antozzi C, Andreetta F, Evoli A, Deymeer F, Saruhan-Direskeneli G, Durmus H, Brenner T, Vaknin A, Berrih-Aknin S, Cuvelier MF, Stojkovic T, DeBaets M, Losen M, Martinez-Martinez P, Kleopa KA, Zamba-Papanicolaou E, Kyriakides T, Kostera-Pruszczyk A, Szczudlik P, Szyluk B, Lavrnic D, Basta I, Peric S, Tallaksen C, Maniaol A, Tzartos SJ (2014) A comprehensive analysis of the epidemiology and clinical characteristics of anti-LRP4 in myasthenia gravis. J Autoimmun 52:139–145. https://doi.org/10.1016/j.jaut.2013.12.004
Acknowledgements
We thank Erciyes University DEKAM vivarium and administrative personnel of GENKOK.
Funding
This study was supported by Erciyes University Scientific Research Council- Doctoral Research Grant (#: TDK-2019-9670) to Sabahattin Muhtaroglu.
Author information
Authors and Affiliations
Contributions
EK, AE and SM conceived the study. SM provided funding. EK carried out all the experiments. EK and AE performed first immunizations. EK, AE and NS killed the mice and harvested the organs. AE supervised all the experimental procedures post-killing and flow cytometry operation. NS helped clinical scoring. Data generation and analyses were performed by EK, GZ and AE. Flow cytometric data analyses on FlowJo and generation of flow plots were performed by AE. All the figures of the manuscript were generated by Emel Koseoglu, except Fig. 1, Supplementary Figs. 13, 14, 15, which were generated by AE. Interpretation of data was performed by EK and AE together. The manuscript was written by AE and EK. All the authors read and contributed to the manuscript preparation, critical reading and approved the submitted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koseoglu, E., Sungur, N., Muhtaroglu, S. et al. The Beneficial Clinical Effects of Teriflunomide in Experimental Autoimmune Myasthenia Gravis and the Investigation of the Possible Immunological Mechanisms. Cell Mol Neurobiol 43, 2071–2087 (2023). https://doi.org/10.1007/s10571-022-01286-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-022-01286-5