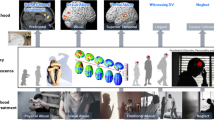
Abstract
The gut–brain axis refers to the bidirectional connection and communication between the gastrointestinal tract and the central nervous system. This paper explores two routes for this communication that have hitherto remained under-examined: epigenetics and redox signaling and their implications for autism spectrum disorder (ASD). The gut microbiota may induce epigenetic changes in the gut and potentially in the brain through their fermentation products. Instead of through other conceptualizations of them acting as neurotransmitters, gut microbial products may act as epigenetic agents, which are supported by the effects of gut bacterial-derived metabolites on gene regulation and expression. In addition to their epigenetic effects, gut bacterial-derived communicative agents can also influence host signaling by contributing to and even substituting host reactive oxygen species (ROS) production. These ROS can act as second messengers and exert oxidative activity on proteins to influence immune, inflammatory, and other signaling processes. ROS and epigenetic mechanisms may have interactive effects as well. ROS, in addition to their role in signaling pathways and cellular redox alterations, also influence redox-sensitive transcription factors, thereby having an effect on gene expression. Specifically, ROS are involved in the activation of transcription factors, chromatin remodeling, and histone/protein deacetylation. These two proposed mechanisms correspond with the recent findings related to ASD, where a cofactor that is shown to be lower in ASD has antioxidative properties, responds to epigenetic modulation, and increases via microbiota interventions. The current evidence reviewed here suggests the need to update models of the gut–brain communication to include these two mechanisms. Such a modeling can also contribute to understanding the unknowns of host metabolism and physiology in ASD and afford potential therapeutic avenues for this as well as other psychiatric and physiological conditions.

Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Aviello G, Knaus UG (2018) NADPH oxidases and ROS signaling in the gastrointestinal tract. Mucosal Immunol 11(4):1011–1023
Bayr H (2005) Reactive oxygen species. Crit Care Med 33(12):S498–S501
Bhattarai Y (2018) Microbiota-gut-brain axis: Interaction of gut microbes and their metabolites with host epithelial barriers. Neurogastroenterol Motil 30(6):e13366
Broderick NA, Buchon N, Lemaitre B (2014) Microbiota-induced changes in Drosophila melanogaster host gene expression and gut morphology. Mbio 5(3):e01117-e1214
Buffington SA, Dooling SW, Sgritta M, Noecker C, Murillo OD, Felice DF et al (2021) Dissecting the contribution of host genetics and the microbiome in complex behaviors. Cell 184(7):1740–1756
Capuco A, Urits I, Hasoon J, Chun R, Gerald B, Wang JK et al (2020) Gut microbiome dysbiosis and depression: a comprehensive review. Curr Pain Headache Rep 24:1–14
Collin F (2019) Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int J Mol Sci 20(10):2407
Cryan JF, O’Riordan KJ, Cowan CS, Sandhu KV, Bastiaanssen TF, Boehme M et al (2019) The microbiota–gut–brain axis. Physiol Rev 99:1877–2013
Cussotto S, Sandhu KV, Dinan TG, Cryan JF (2018) The neuroendocrinology of the microbiota–gut–brain axis: a behavioural perspective. Front Neuroendocrinol 51:80–101
Eshraghi R, Deth RC, Mittal R, Aranke M, Kay SIS, Moshiree B, Eshraghi A (2018) Early disruption of the microbiome leading to decreased antioxidant capacity and epigenetic changes: Implications for the rise in autism. Front Cell Neurosci 12:256
Fellows R, Denizot J, Stellato C, Cuomo A, Jain P, Stoyanova E et al (2018) Microbiota derived short chain fatty acids promote histone crotonylation in the colon through histone deacetylases. Nat Commun 9(1):105
Forman HJ, Maiorino M, Ursini F (2010) Signaling functions of reactive oxygen species. Biochemistry 49(5):835–842
Forman HJ, Ursini F, Maiorino M (2014) An overview of mechanisms of redox signaling. J Mol Cell Cardiol 73:2–9
Frye RE, Huffman LC, Elliott GR (2010) Tetrahydrobiopterin as a novel therapeutic intervention for autism. Neurotherapeutics 7(3):241–249
Fung TC, Olson CA, Hsiao EY (2017) Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci 20(2):145–155
Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D et al (2013) Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504(7480):446
Geirnaert A, Calatayud M, Grootaert C, Laukens D, Devriese S, Smagghe G et al (2017) Butyrate-producing bacteria supplemented in vitro to Crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci Rep 7(1):11450
Gough DR, Cotter TG (2011) Hydrogen peroxide: a Jekyll and Hyde signalling molecule. Cell Death Dis 2(10):e213–e213
Hamilton JP (2011) Epigenetics: principles and practice. Dig Dis 29(2):130–135
Holliday R (2006) Epigenetics: a historical overview. Epigenetics 1(2):76–80
Hooks KB, Konsman JP, O’Malley MA (2019) Microbiota–gut–brain research: a critical analysis. Behav Brain Sci 42:e60
James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, Neubrander JA (2004) Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr 80(6):1611–1617
Jang HM, Lee KE, Kim DH (2019) The preventive and curative effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on immobilization stress-induced anxiety/depression and colitis in mice. Nutrients 11(4):819
Jones RM, Neish AS (2017) Redox signaling mediated by the gut microbiota. Free Radic Biol Med 105:41–47
Kang DW, Adams JB, Coleman DM, Pollard EL, Maldonado J, McDonough-Means S et al (2019) Long-term benefit of microbiota transfer therapy on autism symptoms and gut microbiota. Sci Rep 9(1):1–9
Kelly D, Kotliar M, Woo V, Jagannathan S, Whitt J, Moncivaiz J et al (2018) Microbiota-sensitive epigenetic signature predicts inflammation in Crohn’s disease. JCI Insight 3(18):e122104
Klaiman C, Huffman L, Masaki L, Elliott GR (2013) Tetrahydrobiopterin as a treatment for autism spectrum disorders: a double-blind, placebo-controlled trial. J Child Adolesc Psychopharmacol 23(5):320–328
Knaus UG, Hertzberger R, Pircalabioru GG, Yousefi SPM, Branco dos Santos F (2017) Pathogen control at the intestinal mucosa–H2O2 to the rescue. Gut Microbes 8(1):67–74
Kojima S, Ona S, Iizuka I, Arai T, Mori H, Kubota K (1995) Antioxidative activity of 5, 6, 7, 8-tetrahydrobiopterin and its inhibitory effect on paraquat-induced cell toxicity in cultured rat hepatocytes. Free Radic Res 23(5):419–430
Laurindo FR (2018) Redox cellular signaling pathways in endothelial dysfunction and vascular disease. In: Endothelium and cardiovascular diseases. Academic Press, pp 127–145
Liu S, Gao J, Zhu M, Liu K, Zhang HL (2020) Gut microbiota and dysbiosis in Alzheimer’s disease: Implications for pathogenesis and treatment. Mol Neurobiol 57(12):5026–5043
Mardinoglu A, Shoaie S, Bergentall M, Ghaffari P, Zhang C, Larsson E et al (2015) The gut microbiota modulates host amino acid and glutathione metabolism in mice. Mol Syst Biol 11(10):834
Minato T, Maeda T, Fujisawa Y, Tsuji H, Nomoto K, Ohno K, Hirayama M (2017) Progression of Parkinson’s disease is associated with gut dysbiosis: two-year follow-up study. PLoS ONE 12(11):e1087307
Nishiwaki H, Ito M, Ishida T, Hamaguchi T, Maeda T, Kashihara K et al (2020) Meta-analysis of gut dysbiosis in Parkinson’s disease. Mov Disord 35(9):1626–1635
Noto D, Miyake S (2020) Gut dysbiosis and multiple sclerosis. Clin Immunol. https://doi.org/10.1016/j.clim.2020.108380
Rhee SG (1999) Redox signaling: Hydrogen peroxide as intracellular messenger. Exp Mol Med 31(2):53
Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24(10):R453–R462
Sherwin E, Dinan TG, Cryan JF (2018) Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann N Y Acad Sci 1420(1):5–25
Stilling RM, Dinan TG, Cryan JF (2014) Microbial genes, brain & behaviour–epigenetic regulation of the gut–brain axis. Genes Brain Behav 13(1):69–86
Stone JR, Yang S (2006) Hydrogen peroxide: A signaling messenger. Antioxid Redox Signal 8(3–4):243–270
Sundar IK, Caito S, Yao H, Rahman I (2010) Oxidative stress, thiol redox signaling methods in epigenetics. Methods Enzymol 474:213–244
van der Knaap JA, Verrijzer CP (2016) Undercover: gene control by metabolites and metabolic enzymes. Genes Dev 30(21):2345–2369
Voltan S, Martines D, Elli M, Brun P, Longo S, Porzionato A et al (2008) Lactobacillus crispatus M247-derived H2O2 acts as a signal transducing molecule activating peroxisome proliferator activated receptor-γ in the intestinal mucosa. Gastroenterology 135(4):1216–1227
Vuong HE, Hsiao EY (2017) Emerging roles for the gut microbiome in autism spectrum disorder. Biol Psychiat 81(5):411–423
Wang X, Sun G, Feng T, Zhang J, Huang X, Wang T et al (2019) Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res 29(10):787–803
Zhong GC, Zhao ZB, Cheng Y, Wang YB, Qiu C, Mao LH et al (2021) Epigenetic silencing of GCH1 promotes hepatocellular carcinoma growth by activating superoxide anion-mediated ASK1/p38 signaling via inhibiting tetrahydrobiopterin de novo biosynthesis. Free Radic Biol Med 168:81–94
Acknowledgements
I extend my deepest gratitude to Prof. Fuat Balcı for his invaluable guidance, support, and recommendations about the content of the manuscript and the creation of the figure. I am extremely grateful to Mr. Philip Gee for his careful proofreading and comments on the manuscript.
Funding
No funding was received for this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Doenyas, C. Potential Role of Epigenetics and Redox Signaling in the Gut–Brain Communication and the Case of Autism Spectrum Disorder. Cell Mol Neurobiol 42, 483–487 (2022). https://doi.org/10.1007/s10571-021-01167-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-021-01167-3