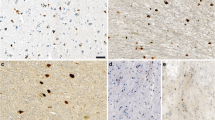
1. Alpha-synuclein is known to play an important role in the pathogenesis of Parkinson’s disease (PD). The pathogenicity of α-synuclein is related to its ability to form intraneuronal inclusions. The inclusions, which are found in brains of patients with PD and diffuse Lewy body disease consist partially of C-terminally truncated α-synuclein. This α-synuclein species has an increased ability to form aggregates compared to full length α-synuclein.
2. We have used an adeno-associated virus (AAV) vector system to overexpress either C-terminally truncated or full length α-synuclein containing the A53T mutation, which have both been identified in brains of familial PD patients and transgenic mouse models. Dissociated mesencephalic neurons, cerebellar granule neurons, and organotypic midbrain slice cultures were infected with AAV containing the transgene under the control of the cytomegalovirus promoter.
3. We demonstrate that viral overexpression of α-synuclein(A53T) leads to the formation of distorted neurites, intraneuritic swellings, and granular perikaryal deposits in cultured neurons. Our results indicate that these cell culture models may represent an early phase of PD reflecting pathologic neuritic alterations before significant neuronal cell loss occurs.



Similar content being viewed by others
Abbreviations
- AAV:
-
adeno-associated virus
- AD:
-
Alzheimer’s disease
- CGN:
-
cerebellar granule neuron
- CMV:
-
cytomegalovirus
- CSPα:
-
cystein-string-protein-α
- DA:
-
dopaminergic
- EGFP:
-
enhanced green fluorescent protein
- HBSS:
-
Hank’s balanced salt solution
- PD:
-
Parkinson’s disease
- TUNEL:
-
terminal deoxyribonucleotidyl transferase (TdT)-mediated biotin-16-dUTP nick-end labeling
- WT:
-
wild-type.
REFERENCES
Bianco, C. L., Ridet, J. L., Dchneider, B. L., Deglon, N., and Aebischer, P. (2002). α-Synuclein and selective dopaminergic neuron loss in a rat lentiviral model of Parkinson’s disease. PNAS 99:10813–10818.
Bottenstein, J., Skaper, S., Varon, S., and Sato, G. (1980). Selective survival of neurons from chick embryo sensory ganglionic dissociates utilizing serum-free supplemented medium. Exp. Cell. Res. 30:183–190.
Braak, H., Ghebremedhin, E., Rüb, U., Bratzke, H., and Del Tredici, K. (2004). Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 318:121–134.
Chandra, S., Gallardo, G., Fernández-Chacón, R., Schlüter, O. M., and Südhof, T. C. (2005). α-Synuclein cooperates with CSPa in preventing neurodegeneration. Cell 123:383–396.
Dong, Z., Ferger, B., Feldon, J., and Büeler, H. (2002). Overexpression of Parkinson’s disease-associated α-synuclein(A53T) by recombinant adeno-associated virus in mice does not increase the vulnerability of dopaminergic neurons to MPTP. J. Neurobiol. 53:1–10.
Duda, J. E., Giasson, B. I., Mabon, M. E., Lee, V. Y., and Trojanowski, J. Q. (2002). Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Ann. Neurol. 52:205–210.
Ehrengruber, M. U., Hennou, S., Büeler, H., Naim, H. Y., Déglon, N., and Lundstrom, K. (2001). Gene transfer into neurons from hippocampal slices: Comparison of recombinant semliki forest virus, adenovirus, adeno-associated virus, lentivirus, and measles virus. Mol. Cell. Neurosci. 17:855–871.
Jensen, P. H., Islam, K., Kenney, J., Nielsen, M. S., Power, J., and Gai, W. P. (2000). Microtubule-associated protein 1B is a component of cortical Lewy Bodies and binds α-synuclein filaments. J. Biol. Chem. 275:21500–21507.
Kirik, D., Rosenblad, C., Burger, C., Lunberg, C., Johansen, T. E., Muzyczka, N., Mandel, R. J., and Björklund, A. (2002). Parkinson-Like neurodegeneration induced by targeted overexpression of α-synuclein in the nigrostratial system. J. Neurosci. 22:2780–2791.
Kontopolous, E., Parvin, J. D., Feany, M. B. (2006). α-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum. Mol. Genet. 15(20):3012–3023.
Kotzbauer, P. T., Giasson, B. I., Kravitz, A. V., Golbe, L. I., Mark, M. H., Trojanowski, J. Q., and Lee, V. M. (2004). Fibrillization of α-synuclein and tau in familial Parkinson’s disease caused by the A53T α-synuclein mutation. Exp. Neurol. 18:279–288.
Krieglstein, K., Suter-Crazzolara, C., Fischer, W. H., and Unsicker, K. (1995). TGF-β superfamily members promote survival of midbrain dopaminergic neurons and protect them against MPP+ toxicity. EMBO J. 14:736–742.
Krüger, R., Kuhn, W., Muller, T., Woitalla, D., Graeber, M., Kosel, S., Przuntek, H., Epplen, J. T., Schols, L., and Riess, O. (1998). Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat. Genet. 18(2):106–108.
Kumlesh, K. D., Hofele, K., Barbieri, S., Buchman, V. L., and Van Der Putten, H. (2003). Part II: α-synuclein and its molecular pathophysiological role in neurodegenerative disease. Neuropharmacology 45:14–44.
Li, W., West, N., Colla, E., Pletnikova, O., Troncoso, J. C., Marsh, L., Dawson, T. M., Jäkälä, P., Hartmann, T., Price, D. L., and Lee, M. K. (2005). Aggregation promoting C-terminal truncation of alpha-synuclein is a normal cellular process and is enhanced by the familial Parkinson’s disease-linked mutations. PNAS 102:2162–2167.
Liu, C. W., Giasson, B. I., Lewis, K. A., Lee, V. M., DeMartino, G. N., and Thomas, P. J. (2005). A precipitating role for truncated α-synuclein and the proteasome in α-synuclein aggregation. J. Biol. Chem. 280:22670–22678.
Machida, Y., Chiba, T., Takayanagi, A., Tanaka, Y., Asanuma, M., Ogawa, N., Koyama, A., Iwatsubo, T., Ito, S., Jansen, P. H., Shimizu, N., Tanaka, K., Mizuno, Y., and Hattori, N. (2005). Common anti-apoptotic roles of parkin and α-synuclein in human dopaminergic cells. Biochem. Biophys. Res. Commun. 332:233–240.
Maingay, M., Romero-Ramos, M., Carta, M., and Kirik, D. (2006). Ventral tegmental area dopamine neurons are resistant to human mutant alpha-synuclein overexpression. Neurobiol. Dis. 23:522–532.
Martin, L. J., Pan, Y., Price, A. C., Sterling, W., Copeland, N. G., Jenkins, N. A., Price, D. L., and Lee, M. K. (2006). Parkinson’s disease α-Synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J. Neurosci. 26:41–50.
Narhi, L., Wood, S. J., Steavenson, S., Jiang, Y., Wu, G. M., Anafi, D., Kaufman, S. A., Martin, F., Sitney, K., Denis, P., Louis, J. C., Wypych, J., Biere, A. L., and Citron, M. (1999). Both familial Parkinson’s disease mutations accelerate alpha-synuclein aggregation. J. Biol. Chem. 274:9843–9846.
Neve, R. L., Neve, K. A., Nestler, E. J., and Carlezon, W. A. (2005). Use of herpes virus amplicon vectors to study brain disorders. Biotechniques 39:381–391.
Panday, N., Schmidt, R. E., and Galvin, J. E. (2006). The alpha-synuclein mutation E46K promotes aggregation in cultured cells. Exp. Neurol. 197:515–520.
Petrucelli, L., O’Farrell, C., Lockhart, P. J., Baptsita, M., Kehoe, K., Vink, L., Choi, P., Wolozin, B., Farrer, M., Hardy, J., and Cookson, M. R. (2002). Parkin protects against the toxicity associated with mutant α-synuclein: Proteasome dysfunction selectively affects catecholaminergic neurons. Neuron 36:1007–1019.
Polymeropoulos, M. H., Lavedan, C., Leroy, E., Die, S. E., Dehejia, A., Dutra, A., Pike, B., Root, H., Rubensstain, J., Boyer, R., Stenroos, E. S., Chandrasekharappa, S., Athanassiadou, A., Papapetropoulos, T., Johnson, W. G., Lazzarini, A. M., Duvoisin, R. C., Di Iorio, G., Golbe, L. I., and Nussbaum, R. L. (1997). Mutation in the alpha-synuclein gene identified in families with Parkinsons’s disease. Science 276:2045–2047.
Quist, A., Doudeviski, I., Lin, H., Azimova, R., Ng, D., Frangione, B., Kagan, B., Ghiso, J., and Lal, R. (2005). Amyloid ion channels: A common structural link for protein-misfolding disease. PNAS 102:10427–10432.
Raisler, B. J., Berns, K. I., Grant, M. B., Beliaev, D., and Hauswirth, W. W. (2002). Adeno-associated virus type-2 expression of pigmented epithelium-derived factor or Kringles 1–3 of angiostatin reduce retinal neovascularisation. Proc. Natl. Acad. Sci. U.S.A. 99:8909–8914.
Shibata, H., Katsuki, H., Nishiwaki, M., Kume, T., Kaneko, S., and Akaike, A. (2003). Lipopolysaccharide-induced dopaminergic cell death in rat midbrain slice cultures: Role of inducible nitric oxide synthase and protection by indomethcin. J. Neurochem. 86:1201–1212.
Shults, C. W. (2006). Lewy bodies. PNAS 103:1661–1668.
Singleton, A. B., Farrer, M., Johnson, J., Singleton, A., Hague, S., Kachergus, J., Hulihan, M., Peuralinna, T., Dutra, A., Nussbaum, R., Lincoln, S., Crawley, A., Hanson, M., Maraganore, D., Adler, C., Cookson, M. R., Muenter, M., Baptista, M., Miller, D., Blancato, J., Hardy, J., and Gwinn-Hardy, K. (2003). Alpha-synuclein locus triplication causes Parkinson’s Disease. Science 302:841.
Spillantini, M. G., Schmidt, M. L., Lee, V. M., Trojanowski, J. Q., Jakes, R., and Goedert, M. (1997). Alpha synuclein in Lewy bodies. Nature 388:839–840.
Stokin, G. B., Lillo, C., Falzone, T. L., Brusch, R. G., Rockenstein, E., Mount, S. L., Raman, R., Davies, P., Masliah, E., Williams, D. S., and Goldstein, L. S. B. (2005). Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science 307:1282–1288.
Stoppini, L., Buchs, P. A., and Muller, D. (1991). A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods 37:173–182.
Tofaris, G. K., Reitböck, P. G., Humby, T., Lambourne, S. L., O’Connell, M., Ghetti, B., Gossage, H., Emson, P. C., Wilkinson, L. S., Goedert, M., and Spillantini, M. G. (2006). Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1–120): Implications for Lewy body disorders. J. Neurosci. 26:3942–3950.
Wilkemeyer, M. F., Smith, K. L., Zarei, M. M., Benke, T. A., Swann, J. W., Angelides, K. J., and Eisensmith, R. C. (1996). Adenovirus-mediated gene transfer into dissociated and explant cultures of rat hippocampal neurons. J. Neurosci. Res. 43:161–174.
Yamada, M., Iwatsubo, T., Mizuno, Y., and Mochizuk, I. H. (2004). Overexpression of α-synuclein in rat substantia nigra results in loss of dopaminergic neurons, phophorylation of α-synuclein and activation of caspase-9: Resemblance to pathogenetic changes in Parkinson’s disease. J. Neurochem. 91:451–461.
Yamaguchi, K., Chochran, E. J., Murell, J. R., Polymeropolous, M. H., Shannon, K. M., Crowther, R. A., Goedert, M., and Ghetti, B. (2005). Abundant neuritic inclusions and microvacuolar changes in a case of diffuse Lewy body disease with the A53T mutation in the alpha-synuclein gene. Acta Neuropathol. 110:298–305.
Zhou, W., Hurlbert, M. S., Schaack, J., Prasdad, K. N., and Freed, C. R. (2000). Overexpression of human α-synuclein causes dopamine neuron death in rat primary culture and immortalized mesencephalon-derived cells. Brain Res. 866:33–43.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zach, S., Bueler, H., Hengerer, B. et al. Predominant Neuritic Pathology Induced by Viral Overexpression of α-Synuclein in Cell Culture. Cell Mol Neurobiol 27, 505–515 (2007). https://doi.org/10.1007/s10571-007-9141-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-007-9141-5