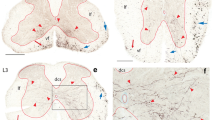
1. The aim of the present study was to examine the distribution of unmyelinated, small-diameter myelinated neuronal nitric oxide synthase immunoreactive (nNOS-IR) axons and large-diameter myelinated neuronal nitric oxide synthase and parvalbumin-immunoreactive (PV-IR) axons in the dorsal funiculus (DF) of sacral (S1–S3) and lumbar (L1–L7) segments of the dog.
2. nNOS and PV immunohistochemical methods were used to demonstrate the presence of nNOS-IR and PV-IR in the large-diameter myelinated, presumed to be proprioceptive, axons in the DF along the lumbosacral segments.
3. Fiber size and density of nNOS-IR and PV-IR axons were used to compartmentalize the DF into five compartments (CI–CV). The first compartment (CI) localized in the lateralmost part of the DF, containing both unmyelinated and small-diameter myelinated nNOS-IR axons, is homologous with the dorsolateral fasciculus, or Lissauer tract. The second compartment (CII) having similar fiber organization as CI is situated more medially in sacral segments. Rostrally, in lower lumbar segments, CII moves more medially, and at upper lumbar level, CII reaches the dorsomedial angle of the DF and fuses with axons of CIV. CIII is the largest in the DF and the only one containing large-diameter myelinated nNOS-IR and PV-IR axons. The largest nNOS-IR and PV-IR axons of CIII (8.0–9.2 μm in diameter), presumed to be stem Ia proprioceptive afferents, are located in the deep portion of the DF close to the dorsal and dorsomedial border of the dorsal horn. The CIV compartment varies in shape, appearing first as a small triangular area in S3 and S2 segments, homologous with the Philippe–Gombault triangle. Beginning at S1 level, CIV acquires a more elongated shape and is seen throughout the lumbar segments as a narrow band of fibers extending just below the dorsal median septum in approximately upper two-thirds of the DF. The CV is located in the basal part of the DF. In general, CV is poor in nNOS-IR fibers; among them solitary PV-IR fibers are seen.
4. The analysis of the control material and the degeneration of the large- and medium-caliber nNOS-IR fibers after unilateral L7 and S1 dorsal rhizotomy confirmed that large-caliber nNOS-IR and and PV-IR axons, presumed to be proprioceptive Ia axons, and their ascending and descending collaterals are present in large number in the DF of the lumbosacral intumescence. However, in the DF of the upper lumbar segments, the decrease in the number of nNOS-IR and PV-IR fibers is quite evident.












Similar content being viewed by others
Abbreviations
- CI–CV:
-
fibre compartments in DF
- DF:
-
dorsal funiculus
- DRG:
-
dorsal root ganglion
- DREZ-one:
-
dorsal root entry zone
- eNOS:
-
endothelial nitric oxide synthase
- LB:
-
lateral bundle of the dorsal root
- LS:
-
lumbosacral segments
- MB:
-
medial bundle of the dorsal root
- mNOS:
-
macrophage nitric oxide synthase
- NADPHd:
-
nicotinamide adenine dinucleotide phosphate diaphorase
- nNOS-IR:
-
neuronal nitric oxide synthase immunoreactivity
- PV-IR:
-
parvalbumin immunoreactivity
- TPG:
-
the triangle of Philippe–Gombault.
REFERENCES
Altman, J., and Bayer, S. A. (2001). Development of the Human Spinal Cord. An Interpretation Based on Experimental Studies in Animals. Oxford University Press, Oxford.
Besson, J. M., and Chaouch, A. (1987). Peripheral and spinal mechanisms of nociception. Physiol. Rev. 67:67–186.
Bredt, D. S., Hwang, P. M., and Snyder, S. H. (1990). Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature 347:768–770.
Brodal, A. (1969). Neurological Anatomy. Oxford University Press, New York.
Brown, A. G., and Fyffe, R. E. (1978). The morphology of group Ia afferent fiber collaterals in the spinal cord of the cat. J. Physiol. 274:111–127.
Brown, A. G., and Fyffe, R. E. (1981). Direct observations on the contacts made between Ia afferent fibres and alpha-motoneurones in the cat’s lumbosacral spinal cord. J. Physiol. 313:121–140.
Brown, A. G. (1981). Organization in the Spinal Cord: The Anatomy and Physiology of Identified Neurones. Springer-Verlag, Berlin.
Celio, M. R. (1990). Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience 35:375–475.
Chung, K., and Coggeshall, R. E. (1985). Unmyelinated primary afferent fibers in dorsal funiculi of cat sacral spinal cord. J. Comp. Neurol. 238:365–369.
Chung, K. S., and Coggeshall, R. E. (1987). Postnatal development of the rat dorsal funiculus. J. Neurosci. 7:972–977.
Chung, K., Langford, L. A., and Coggeshall, R. E. (1987). Primary afferent and propriospinal fibers in the rat dorsal and dorsolateral funiculi. J. Comp. Neurol. 263:68–75.
Chung, K., Sharma, J., and Coggeshall, R. E. (1985). Numbers of myelinated and unmyelinated axons in the dorsal, lateral, and ventral funiculi of the white matter of the S2 segment of cat spinal cord. J. Comp. Neurol. 234:117–121.
Clowry, G. J., Arnott, G. A., Clement-Jones, M., Fallah, Z., Gould, S., and Wright, C. (2000). Changing pattern of expression of parvalbumin immunoreactivity during human fetal spinal cord development. J. Comp. Neurol. 423:727–735.
Clowry, G. J., Moss, J. A., and Clough, R. L. (2005). An immunohistochemical study of the development of sensorimotor components of the early fetal human spinal cord. J. Anat. 207:313–324.
Dekkers, J., Greensmith, L., and Navarrete, R. (2002). Changes in the expression of parvalbumin immunoreactivity in the lumbar spinal cord of the rat following neonatal nerve injury. Dev. Neurosci. 24:283–293.
Dinerman, J. L., Dawson, T. M., Schell, M. J., Snowman, A., and Snyder, S. H. (1994). Endothelial nitric oxide synthase localized to hippocampal pyramidal cells: Implications for synaptic plasticity. Proc. Natl. Acad. Sci. USA 91:4214–4218.
Fyffe, R. E. W. (1979). The morphology of Group II muscle afferent fiber collaterals. J. Physiol. 296:39–40.
Fyffe, R. E. W., and Light, A. R. (1984). The ultrastructure of group Ia afferent fiber synapses in the lumbosacral spinal cord of the cat. Brain Res. 300:201–209.
Hirshberg, R. M., Al-Chaer, E. D., Lawand, N. B., Westlund, K. N., and Willis, W. D. (1996). Is there pathway in the posterior funiculus that signals visceral pain? Pain 67:291–305.
Hongo, T., Kudo, N., Sasaki, S., Yamashita, M., Yoshida, K., Ishizuka, N., and Mannen, H. (1987). Trajectory of group Ia and Ib fibers from the hind-limb muscles at the L3 and L4 segments of the spinal cord of the cat. J. Comp. Neurol. 262:159–194.
Ishizuka, N., Mannen, H., Hongo, T., and Sasaki, S. (1979). Trajectory of group Ia afferent fibers stained with horseradish peroxidase in the lumbosacral spinal cord of the cat: Three dimensional reconstructions from serial sections. J. Comp. Neurol. 186:189–212.
Knyihár-Csillik, E., Rakic, P., and Csillik, B. (1999). Illusive transience of parvalbumin expression during embryonic development of the primate spinal cord. Int. J. Dev. Neurosci. 17:79–97.
Kudo, N., and Yamada, T. (1987). Morphological and physiological studies of development of the monosynaptic reflex pathway in the rat lumbar spinal cord. J. Physiol. 389:441–459.
Lazorthes, Y., Sol, J. C., Sallerin, B., and Verdié, J. C. (2002). The surgical management of spasticity. Europ. J. Neurol. 9:35–41.
Lukáčová, N., Čížková, D., Maršala, M., Lukáč, I., and Maršala, J. (2002). The regional distribution of nitric oxide synthase activity in the spinal cord of the dog. Brain Res. Bull. 58:173–178.
Lukáčová, N., Kolesár, D., Maršala, M., and Maršala, J. (2006). Immunohistochemical, histochemical and radioassay analysis of nitric oxide synthase immunoreactivity in the lumbar and sacral dorsal root ganglia of the dog. Cell. Mol. Neurobiol. 26:17–44.
Maršala, J., Maršala, M., Vanický, I., and Taira, Y. (1999). Localization of NADPHd-exhibiting neurons in the spinal cord of the rabbit. J. Comp. Neurol. 406:263–284.
Maršala, J., Lukáčová, N., Čížková, D., Kafka, J., Katsube, N., Kuchárová, K., and Maršala, M. (2002). The case for the bulbospinal respiratory nitric oxide synthase-immunoreactive pathway in the dog. Exp. Neurol. 177:115–132.
Maršala, J., Kluchová, D., and Maršala, M. (1997). Spinal cord gray matter layers rich in NADPH diaphorase-positive neurons are refractory to ischemia-reperfusion-induced injury: a histochemical and silver impregnation study in rabbit. Exp. Neurol. 145:165–179.
Maršala, J., Lukáčová, N., Čížková, D., Lukáč, I., Kuchárová, K., and Maršala, M. (2004). Premotor nitric oxide synthase immunoreactive pathway connecting lumbar segments with the ventral motor nucleus of the cervical enlargement in the dog. J. Chem. Neuroanat. 27:43–54.
Maršala, J., Lukáčová, N., Kolesár, D., Kuchárová, K., and Maršala, M. (2006). Nitrergic proprioceptive afferents originating from quadriceps femoris muscle are related to monosynaptic Ia-motoneuron stretch reflex circuit in the dog. Cell. Mol. Neurobiol. 26:1385–1410.
Maršala, J., Lukáčová, N., Šulla, I., Wohlfahrt, P., and Maršala, M. (2005). The evidence for nitric oxide synthase immunopositivity in the monosynaptic Ia-motoneuron pathway of the dog. Exp. Neurol. 195:161–178.
Maršala, J., Maršala, M., Lukáčová, N., Ishikawa, T., and Čížková, D. (2003). Localization and distribution patterns of nicotinamide adenine dinucleotide phosphate diaphorase exhibiting axons in the white matter of the spinal cord of the rabbit. Cell. Mol. Neurobiol. 23:57–92.
Maršala, J., Šulla, I., Jalč, P., and Orendáčová, J. (1995). Multiple protracted cauda equina constrictions cause deep derangement in the lumbosacral spinal cord circuitry in the dog. Neurosci. Lett. 193:97–100.
Maršala, J., Vanický, I., Maršala, M., Jalč, P., Orendáčová, J., and Taira, Y. (1998). Reduced nicotinamide adenine dinucleotide phosphate diaphorase in the spinal cord of dogs. Neuroscience 85:847– 862.
Maxwell, D. J., and Bannatyne, B. A. (1983). Ultrastructure of muscle spindle afferent terminations in lamina VI of the cat spinal cord. Brain Res. 288:297–301.
Maxwell, D. J., Christie, W. M., Ottersen, O. P., and Storm-Mathisen, J. (1990). Terminals of group Ia primary afferent fibers in Clarkes column are enriched with L-glutamate-like immunoreactivity. Brain Res. 510:346–350.
McKenna, K. E., and Nadelhaft, I. (1986). The organization of the pudendal nerve in the male and female rat. J. Comp. Neurol. 22:248:532–549.
Molander, C., Ygge, J., and Dalsgaard, C. J. (1987). Substance P-, somatostatin- and calcitonin gene-related peptide-like immunoreactivity and fluoride resistant acid phosphatase-activity in relation to retrogradely labeled cutaneous, muscular and visceral primary sensory neurons in the rat. Neurosci. Lett. 74:37–42.
Nicol, M. J., and Walmsley, B. (1991). A serial section electron microscope study of an identified Ia afferent collateral in the cat spinal cord. J. Comp. Neurol. 314:257–277.
Orendáčová, J., Čížková, D., Kafka, J., Lukáčová, N., Maršala, M., Sulla, I., Maršala, J., and Katsube, N. (2001). Cauda equina syndrome. Prog. Neurobiol. 64:613–637.
Orendáčová, J., Maršala, M., Sulla, I., Kafka, J., Jalč, P., Čížková, D., Taira, Y., and Maršala, J. (2000). Incipient cauda equina syndrome as a model of somatovisceral pain in dogs: Spinal cord structures involved as revealed by the expression of c-fos and NADPH diaphorase activity. Neuroscience 95:543–557.
Patterson, J. T., Coggeshall, R. E., Lee, W. T., and Chung, K. (1990). Long ascending unmyelinated primary afferent axons in the rat dorsal column: Immunohistochemical localizations. Neurosci. Lett. 108:6–10.
Patterson, J. T., Chung, K., and Coggeshall, R. E. (1992). Further evidence for the existence of long ascending unmyelinated primary afferent fibers within the dorsal funiculus: effects of capsaicin. Pain 49:117–120.
Pearson, A. A. (1952). Role of gelatinous substance of spinal cord in conduction of pain. Arch. Neurol. Psych. 68:515–529.
Pierce, J. P., and Mendell, L. M. (1993). Quantitative ultrastructure of Ia boutons in the ventral horn: Scaling and positional relationships. J. Neurosci. 13:4748–4763.
Ranson, S. W. (1914). An experimental study of Lissauer's tract and the dorsal roots. J. Comp. Neurol. 24:531–545.
Sherrington, C. S. (1906). The Integrative Action of the Nervous System. Yale University Press, New Haven.
Sindou, M., Quoex, C., and Baleydier, C. (1974). Fiber organization at the posterior spinal cord-rootlet junction in man. J. Comp. Neurol. 153:15–26.
Snyder, R. (1977). The organization of the dorsal root entry zone in cats and monkeys. J. Comp. Neurol. 174:47–70.
Uddenberg, N. (1968). Functional organization of long, second-order afferents in the dorsal funiculus. Exp. Brain Res. 4:377–382.
Walmsley, B., Wieniawa-Narkiewicz, E., and Nicol, M. J. (1985). The ultrastructural basis for synaptic transmission between primary muscle afferents and neurons in Clarke’s column of the cat. J. Neurosci. 5:2095–2106.
White, J. C., and Sweet, W. H. (1969). Pain and Neurosurgeon. Thomas, Springfield, IL.
Wiklund, N. P., Cellek, S., Leone, A. M., Iversen, H. H., Gustafsson, L. E., Brundin, L., Furst, V. W., Flock, A., and Moncada, S. (1997). Visualisation of nitric oxide released by nerve stimulation. J. Neurosci. Res. 47:224–232.
Wiklund, N. P., Iversen, H. H., Leone, A. M., Cellek, S., Brundin, L., Gustafsson, L. E., and Moncada, S. (1999). Visualization of nitric oxide formation in cell cultures and living tissue. Acta Physiol. Scand. 167:161–166.
Willis, W. D., Al-Chaer, E. D., Quast, M. J., and Westlund, K. N. (1999). A visceral pain pathway in the dorsal column of the spinal cord. Proc. Natl. Acad. Sci. USA 96:7675–7679.
Willis, W. D., and Coggeshall, R. E. (1991). Sensory Mechanisms of the Spinal Cord, Plenum Press, New York.
Willis, W. D., and Westlund, K. N. (1997). Neuroanatomy of the pain system and of the pathways that modulate pain. J. Clinic. Neurophysiol. 14:2–31.
Zhang, J. H., Morita, Y., Hironaka, T., Emson, P. C., and Tohyama, M. (1990). Ontological study of calbindin-D28k-like and parvalbumin-like immunoreactivities in rat spinal cord and dorsal root ganglia. J. Comp. Neurol. 302:715–728.
Acknowledgments
The authors thank Mr. D. Krokavec, Ms. M. Špontáková, Mrs. M. Syneková, and Mrs. A.M. Košová for their excellent technical assistance. The experimental work was supported by the VEGA Grants No. 2/3217/23 and 2/5134/25 from the SAS, APVT Grant No. 51-013002, and NIH grants NS 32794 and NS 40386 to M.M.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maršala, J., Lukáčová, N., Kolesár, D. et al. The Distribution of Primary Nitric Oxide Synthase- and Parvalbumin- Immunoreactive Afferents in the Dorsal Funiculus of the Lumbosacral Spinal Cord in a Dog. Cell Mol Neurobiol 27, 475–504 (2007). https://doi.org/10.1007/s10571-007-9140-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-007-9140-6