Abstract
The paper presents results of tests on the possibility of utilising fabric with a deposited spatial network of multiwall carbon nanotubes (MWCNTs) as electrodes in processes of their functionalization by electrochemical methods enabling deposition of metal nanoparticles, as well as—by electropolymerization—of conductive polymer nanocoatings. Cyclic voltammetry was employed in the testing, which was used to functionalise various types of fabrics covered with a MWCNT spatial network by depositing silver nanoparticles and polypyrrole (PPy) nanocoating, conferring bioactive properties and significantly increased specific capacity, respectively, to the layered hybrid textile materials acquired this way.
Similar content being viewed by others
Introduction
The specific properties of carbon nanotubes (CNT), including in particular—for economic reasons—multiwalled carbon nanotubes (MWCNT), inspired a great global interest of research centres. This interest translates into research on an equal scale, concerning methods and technologies of producing various types of materials modified with MWCNTs finding increasingly wider and more varied industrial applications. The measure of world-wide interest of research centres in MWCNTs and of the intensiveness of research conducted in this field, as well as the ever broader areas of carbon nanotube applications, is a very rich body of literature, both scientific and patent-related. MWCNTs have found wide applications in production of modern composite materials, mainly used for manufacturing new generations of electric and electronic equipment, but also in other branches of industry, e.g. in automotive industry. For example, such composites are used for manufacturing electricity storage equipment (Kim et al. 2001; Wang et al. 2011), biosensors and sensors (Bartlett and Cooper 1993; Foulds and Lowe 1986), and in electrochemistry—electrochemical capacitors (Jurewicz et al. 2001; Zhang et al. 2004) or fuel cells (Yuan et al. 2013; Zou et al. 2008), characterized by high hydrogen adsorption/storage capacity. An increasing interest is also found in carbon nanotube doping of various plastics, enabling major improvement in their electric and mechanical properties (Devi et al. 2012; Foroughi et al. 2010; Iijima 1991; Treacy et al. 1996). Possibilities of further optimization of properties of this type of composite materials are predicted in functionalization of MWCNTs used for plastics doping. Research in this area is conducted in numerous institutions around the world. The possibility of functionalizing MWCNTs was tested for instance by their deposition on the surface of metal nanoparticles, metal oxides or inter-chain conductive polymer coatings in order to obtain composites with bioactive and catalytic properties, respectively (Sadeghnejad et al. 2014), or ones intended for electrodes in supercapacitors (Jurewicz et al. 2001; Zhang et al. 2004).
The newest, intensively studied type of MWCNT-modified materials are multifunctional hybrid materials, an important area of which covers elastic layered composites with a conductive spatial MWCNT network deposited on a textile substrate—on the surfaces of fibres forming appropriate structures, most commonly fabrics (Makowski et al. 2014). Research in this area has only been taken up in recent years, and it mostly concerns fabrics modified using the ‘wet method’, by deep coating with water dispersion of nanotubes with an addition of an appropriate surfactant—dispersant. The necessary condition for effective formation of a conductive MWCNTs network on a textile substrate, which requires reaching the percolation threshold at the lowest nanotube deposition possible, is preparing a nanotube suspension which is homogenous, highly dispersed and as mono-particular as possible (Hock and Harris 1940; Vaisman et al. 2006). Meeting this condition required developing an appropriate procedure of preparing nanotube dispersion used as a deep coating bath. A very high interest in such systems, classified as smart textiles, results from the potential possibility to use them as light and flexible antistatic or conductive materials, heating elements, microwave radiation absorbers, electromagnetic screens, sensors, antennas, etc. A new, highly interesting research field is the possibility to acquire multifunctional, flexible layered composites, but not through deposition of pre-functionalized MWCNTs on textile substrates, but by reverse functionalization of finished layered hybrid materials—fabrics with deposited spatial MWCNTs network.
Fibres forming the fabric structure form a sort of scaffolding, supporting and spatially shaping the nanotube network. Compared to non-immobilised tubes, this creates more options and different conditions for their modification.
However, immobilization methods for the preparation of silver nanoparticles on the surface of the nanotubes CNTs are a lot e.g. through reduction (Sathishkumar et al. 2010), sonochemical method (Perelshtein et al. 2008), crosslinkers (Alimohammadi et al. 2012, 2013; Ravindra et al. 2010), layer by layer deposition (Gorjanc et al. 2010) or UV radiation (Gashti and Almasian 2013; Gashti et al. 2015).
Interesting original results were achieved by depositing appropriate hydrophobising agents, e.g. methylchlorosilane, onto such hybrid materials, resulting in materials with superhydrophobic properties and a specific nanostructure of the fibre surface, which provides such materials with self-cleaning capability (Makowski et al. 2014). The results we obtained justified the continuation of research in this area, particularly studies on the possibility of producing a new type of multifunctional elastic hybrid materials. The basic assumption of this study was the use of fabrics with a deposited spatial conductive nanotube network as electrodes in processes of their functionalization by way of electrochemical methods, enabling deposition of metal nanoparticles or—using electropolymerization—conductive polymer coatings on such MWCNTs networks.
The paper presents the results of our research obtained to date.
Experimental
Textile materials
Cotton woven fabric
A commercial woven fabric of 100 % cotton fibers, with plain weave, a surface weight of 145 g/m2 and a thickness of 0.36 mm. Warp yarn: tex 25, density of warp 295 threads/10 cm, weft yarn: tex 25, density of weft 205 threads/10 cm. The woven fabric was pre-purified/washed under industrial conditions and dried.
Polyester woven fabric
Woven fabric with a plain weave made of multi-filament continuous polyester yarns: warp—PET DTY WP dtex 84f36; weft—PET DTY WP dtex 84f144x2, a surface weight of 93 g/m2 and a thickness of 0.19 mm. The woven fabric was finished according to industrial standard (washing, drying and thermostabilization).
Electrochemistry reagents, dispersing agents and solvents
-
Sodium hypochlorite 13 % active chlorine (≥98 % Avantor, Poland)
-
Silver nitrate (≥99 %, Avantor, Poland)
-
Trisodium citrate (≥99 % Avantor, Poland)
-
Solvent—double distillated H2O (ddH2O)
-
Dispersing agent—SDS—sodiumlauryl sulfate (≥99 % Carl Roth GmbH, Germany)
Carbon nanotubes
The multiwall carbon nanotubes (MWCNTs) Nanocyl NC-7000 with an average diameter of 9.5 nm, a length of 1.5 μm (method of measurement transmission electron microscopy), purity of 90 % and a specific surface of 250–300 m2/g (Nanocyl S.A., Belgium).
Apparatus
Surface morphology, content of elements present in fibers and nanostructure formed
The surface and chemical composition of the analyzed samples were carried out using a scanning electron microscope (SEM—JEOL 5500 Tokyo, Japan) with energy-dispersive X-ray spectroscopy (EDS). Microphotographs were taken after the surface of samples had been coated with a gold layer (5 nm thick) by ion sputtering (Quorum Technologies Ltd—EMS150R ES East Sussex, UK).
Morphological and topographic modifications of the fabrics were performed using an atomic force microscope (AFM). Tests were carried out using a Nanoscope IIIa microscope in tapping mode (TM-AFM) (Digital Instruments, Santa Barbara, USA). Besides topographic images, phase images were also recorded. All tests were conducted in an atmosphere of air at room temperature and at scanning frequency below 1.5 Hz.
Electrodeposition and electropolymerization were carried out using a BioLogic SP-150 (Bio-Logic Science Instruments SAS, Claix, France) potentiostat/galvanostat, utilising a platinum (Pt°) electrode as the counter electrode (CE), and a silver chloride electrode (Ag|AgCl) as the reference electrode (RE) The rate of voltage change over time during each of these phases was 0.020 V/s. EC-Lab Electrochemistry v10.40 software was employed for control and analysis of the process. Polypyrrole nanocoatings formed on the surface of MWCNTs networks deposited on the fabrics were analyzed using a Thermo Scientific Nicolet 380 FT-IR (Thermo Fisher Scientific, Inc. (NYSE:TMO), USA) spectrometer. Infrared spectra in the range 500–4000 cm−1 were recorded with a 1 cm−1 resolution.
Employed methods of test material preparation
Preparation of fabric with a deposited MWCNT network
Preparation processes of cotton woven fabric samples
To completely remove purification residues such as wax, fats and pectins, cotton woven fabric samples were purified by extraction in turn with diethyl ether (98 % analytically pure, CHEMPUR, Poland) and then anhydrous ethanol (analytically pure, CHEMPUR, Poland) for 2 h each.
The fabrics were then additionally purified in boiling ethanol under reflux condenser (2 h) followed by rinsing with doubly distilled water and drying at a temperature of 120 °C for 30 min.
Preparation of aqueous dispersion of MWCNTs
The nanotubes were dispersed in an aqueous solution of SDS (dispersing agent), using of ultrasonic sonotrode (power 200 W, amplitude 30 %, frequency 50/60 Hz). The concentration of MWCNTs in the aqueous dispersion was 0.5 %, and the ratio of MWCNTs to SDS was equal to 1:3.
Deposition of MWCNTs dispersion on woven fabrics
The aqueous dispersion of MWCNTs was deposited on dry cotton or polyester fabric samples by padding method using a laboratory double-roll padding machine with horizontally set squeezing rollers (BENZ KLFH 322 K, Switzerland). The temperature of deep coating bath was 25 °C. The padding rate was 1 m/min with a squeezing rollers pressure amounting to 20 kG/cm along the roller contact line. The saturated woven fabric was then drying at a temperature of 100 °C for 15 min. In order to remove the dispersing agent and excess nanotubes, the samples were several times rinsed in distilled water followed by drying in hot air at 120 °C for 1 h.
Electrochemical deposition of nanosilver (Ag°) on conductive cotton or polyester woven fabric
Electrochemical deposition of Ag° nanoparticles on a fabric surface with deposited carbon nanotubes was performed by three cycles voltammetry in a mixture of solutions of sodium citrate, at a concentration of C1 = 0.1 mol/dm3, and silver nitrate, at a concentration of C2 = 0.002 mol/dm3. These solutions were mixed with at a ratio of 1:1 v/v, and then filtered through a PTFE filter (0.2 μm) and placed in a dark vessel, which was blown through for 45 min with dry, pure nitrogen before use. The fabric with a deposited MWCNT network, secured to a glass galvanostat arm, serves as a specific carbon electrode. The resistance electrode before deposition was 0.26 kΩ/cm2 for cotton and 0.36 kΩ/cm2 for PET, after deposition of resistance was the same. The process was conducted at a room temperature. Apart from the specific carbon electrode, the measurement system also included a counter electrode (CE) in the form of a platinum electrode (Pt°) and a reference electrode (RE) in the form of a silver chloride electrode (Ag|AgCl) (Fig. 1).
Parameters of silver nanoparticle production using the cyclic voltammetry
Silver nanoparticles were formed on the surface of a fabric with a spatial conductive MWCNTs network by cyclic voltammetry. As a result of change in the potential, first towards negative values, down to −1.0 V versus Ag|AgCl, and subsequently towards positive values +1.0 V versus Ag|AgCl, a reduction in silver ions occurred on the surface of the fabric.
Electrochemical deposition of polypyrrole (PPy) nanocoat on conductive cotton or polyester woven fabric
Deposition of polypyrrole coatings (with π-electron conductivity) onto fabrics with a MWNCTs spatial network, obtained by electropolymerization using cyclic voltammetry, aims to increase the capacity of a specific carbon electrode (Pieta et al. 2009). In order to conduct this process, three water solutions were prepared: sodium citrate C1 = 0.45 mol/dm3, pyrrole C2 = 0.1 mol/dm3 and sodium chlorate (I) C3 = 0.20 mol/dm3, which were mixed at a volume ratio of 1:1:1. The solution obtained this way was subject to filtration on a PTFE filter (0.2 μm) and subsequently stored in a dark vessel, pre-blown with pure nitrogen for 45 min. A sample of fabric with a deposited spatial MWCNT network, constituting a specific carbon electrode, was secured to a glass arm, connected to the galvanostat and immersed in the prepared reaction solution/mixture. The resistance electrode before deposition was 0.35 kΩ/cm2 for PET and 0.26 kΩ/cm2 for cotton, after deposition of resistance was 0.36 kΩ/cm2 for PET and 0.27 kΩ/cm2 for cotton. The utilized measurement system, consisting of three electrodes, was identical to the system described above (Electrochemical deposition of nanosilver (Ag°) on conductive woven fabric). Pyrrole electropolymerization was conducted at a room temperature.
Parameters of pyrrole electropolymerisation using cyclic voltammetry
The process of electropolymerization on the surface of fabrics with a spatial conductive MWCNT network was performed by way of cyclic voltammetry. Since polymerization is an anodic process occurring as a result of pyrrole oxidation, deposition of polymer nanocoatings on the electrode surface was achieved by changing the potential towards positive values, up to +1.0 V versus Ag|AgCl, and subsequently towards negative values, −0.8 V versus Ag|AgCl.
Bioactive properties of modified fabrics
The bioactive properties of cotton and polyester fabrics modified by MWCNTs and silver nanoparticles were evaluated against Escherichia coli (Gram negative bacteria) and Staphylococcus aureus (Gram positive bacteria) using disc diffusion method. In this method, discs containing silver nanoparticles were plased on PCA agar (Merck) plates inoculated previously by tested microorganism. All the plates were incubated at 37 °C for 24 h, following which the zone of inhibition was measured (Dickert et al. 1981).
Results and discussion
The process of forming silver nanoparticles on the surface of woven it was the result of electrochemical reduction of silver ions.
The reaction of silver nitrate with sodium citrate produces silver citrate ions stabilized by carboxyl groups of this acid (Patra et al. 2014).
The most effective complexing occurs when [citrate]/[Ag+] ≫ 1 (Fig. 2). Thus formed complex [citrate]/[Ag+] increases the distance between silver ions and prevents aggregation of silver nanoparticles during cyclic voltammetry while the reduction of silver ions on carbon nanotubes forms a conductive spatial network on the fabric surface (Fig. 3). The produced silver nanoparticles were observed on the fabric surface using a scanning electron microscope (SEM). As can be seen in SEM images (Fig. 4), regardless of the fabric type—cotton or polyester—the formed Ag° nanoparticles of uniform size are present on the surface of nanotubes. In order to determine the size of both the silver nanoparticles, produced and deposited on the surface of carbon nanotubes, and of the nanotubes themselves, which form a conductive spatial network on the fabric surface, surface morphology of the obtained hybrid material was analyzed using an atomic force microscope (AFM). A comparison of the MWCNTs surface before and after depositing silver nanoparticles (Figs. 5, 6) provides confirmation of changes on the fabric surface. The images present the fabric surface without (Fig. 5a, b) and with a deposited MWCNTs network. An even network of single nanotubes, without disadvantageous clusters or aggregates, is visible in the microimage (Fig. 5c, d). This indicates a high, monodisperse particles of the nanotube suspension used as a deep coating bath during their deposition on the surface of fibres/fabric. This is possible due to the use of fibers (PET) that have a smooth surface structure (Fig. 5a, b).
Fabrics with a deposited MWCNT network prepared this way were used as electrodes in the reaction of silver ion electrochemical reduction. After performing the reduction reaction, the conductive fabric was washed, dried and subject to surface topography examination using an AFM.
Despite limited density/deposition of carbon nanotubes, a continuous, spatial conductive network of carbon nanotubes is visible on the fibre/fabric surface (Fig. 6).
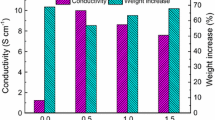
Continuity of the obtained nanotube network is confirmed by the reduction process occurring on the nanotube/silver ion contact surface, combined with nucleation and forming of silver nanoparticles. The height and width of the formed silver nanoparticles, determined using an AFM, was 43 and 95 nm, respectively. At the same time, the average calculated nanotube diameter/size (height) was hMWCNT = 8 nm. It is consistent with information from the nanotubes manufacturer and it indicates a very good dispersion of nanotubes in the deep coating bath. In order to confirm whether the nanotube network surface is covered with silver nanoparticles (or reduced silver ions) an EDS analysis was performed (Fig. 7). The analysis confirmed presence of silver nanoparticles on the surface of the conductive nanotube network on the fabric. The performed analysis indicates that conductive fabrics (with a deposited MWCNTs network) can serve as electrodes in cyclic voltammetry. Performing three cycles of the voltammetry process is sufficient for obtaining hybrid textile materials/fabrics with expected properties. The voltammetric curves during silver particle electrodeposition are presented in (Fig. 8).
The produced conductive fabrics (with a deposited MWCNTs network) were also used as electrodes in pyrrole electropolymerization. The proposed mechanism of this process, which results in forming of a polypyrrole (PPy) coating on the nanotube network surface, is presented in Fig. 9. The electropolymerization was conducted in two stages. Stage one consisted of pyrrole electrodepositing on a platinum electrode (Pt°). This stage was intended to enable selection of appropriate process parameters, i.e. speed, number and range of voltage scanning. Stage two involved forming a PPy coating on the surface of the MWCNTs spatial network and obtaining a composite material with the specified parameters. PPy electropolymerization is presented in Fig. 10. It was observed during the study that a PPy nanocoating on the MWCNTs network surface is obtained after a 6-cycle electropolymerization process. The surface morphology of the obtained layered nanocomposite was examined using scanning electron microscopy (SEM) and atomic force microscopy (AFM) (Fig. 11).
On the obtained images from an electron microscope (SEM) (Fig. 11a) after the electropolymerisation we will notice some differences on the coating of PET fibers. Using atomic force microscope (AFM) has enabled in an unambiguous way to determine the morphology of nanocoatings PPy (Fig. 11b).
Zone 1 on the image (Fig. 11b1) showed ribbons structure. It is the region (probably) responsible for weak signal FTIR derived from conductive polymer (PPy). Zone 2 (Fig. 11b2) showed granular structure. Correctness of the utilised PPy electropolymerisation on the MWCNT surface was verified by the FT-IR method (Fig. 12). The test was performed on a cotton fabric with a deposited MWCNTs network. An FTIR spectrum for composite PPy/Ag° is similar to polypyrrole/silver coated wool (Gashti et al. 2015). Two weak PPy peaks at signals located in the vicinity of the 1532 and 910 cm−1 indicate presence of a conductive polymer in its conductive state (Schmid et al. 2009). However, the latter range is not identical with spectra in the literature, which may indicate that lower coupling of PPy chains was achieved (Avlyanov et al. 1997). No precise location of the PPy absorption band is observed either, as it is broad and lies within the 900–1100 cm−1 range (Manik et al. 2011).
Microbiological activity of fabrics with deposited carbon nanotubes and silver nanoparticles electrochemically deposited on their surface was tested by a qualitative method. Results suggest that both types of fabrics with the embedded method of cyclic voltammetry silver nanoparticles show microbial activity against gram-negative-E. coli, and S. aureus-gram-positive, but this activity is greater in relation to E. coli. A bacteria growth inhibition zone was visible for these samples, as depicted in Figs. 13 (Dickert et al. 1981). The probable mechanism of zone growth inhibition of cotton and polyester woven fabrics with MWCNT and silver nanoparticles is interaction of these particles with intracellular substances causing their coagulation and removal from the liquid system. The silver nanoparticles not only interact with the surface of membrane, but can also penetrate inside the bacteria suggested in the publications (Bryaskova et al. 2011; Sharma et al. 2009; Shrivastava et al. 2007; Sondi and Salopek-Sondi 2004).
Conclusions
Based on the conducted tests, it was concluded that:
-
Cotton and polyester fabrics with a deposited spatial network of carbon nanotubes (MWCNTs) can undergo further bioactive or electric functionalization by using them as electrochemical electrodes in the processes of silver nanoparticle electrodeposition or pyrrole electropolymerization, forming polypyrrole (PPy) nanocoatings.
-
Utilization of the developed electrochemical processes and the cyclic voltammetry method enables functionalization of hybrid materials—fabrics with a deposited MWCNTs network—by forming and nanoparticle depositing of silver nanoparticles or a continuous polypyrrole nanocoating on the MWCNTs network surface.
-
As a result of uniform and nanoparticle electrodeposition of silver nanoparticles on the surface of fabrics with carbon nanotubes deposited, fabrics were obtained with antibacterial properties against E. coli and S. aureus.
-
As a result of pyrrole electropolymerization on the surface of a fabric with an MWCNT network deposited, a conductive polymer—polypyrrole—nanocoating was formed.
-
The functional layered hybrid materials produced using the prepared methods are characterized by flexibility, low surface mass and easy of shaping, which creates potential for their use in the electric and electronic equipment industry.
Achievement
Utilization of electrochemical methods—voltammetry for reverse functionalization of spatial MWCNT networks—electrodeposition of Ag nanoparticles or PPy nanocoatings.
References
Alimohammadi F, Gashti MP, Shamei A (2012) A novel method for coating of carbon nanotube on cellulose fiber using 1,2,3,4-butanetetracarboxylic acid as a cross-linking agent. Prog Org Coat 74:470–478. doi:10.1016/j.porgcoat.2012.01.012
Alimohammadi F, Gashti MP, Shamei A (2013) Functional cellulose fibers via polycarboxylic acid/carbon nanotube composite coating. J Coat Technol Res 10:123–132. doi:10.1007/s11998-012-9429-3
Avlyanov JK, Kuhn HH, Josefowicz JY, MacDiarmid AG (1997) In-situ deposited thin films of polypyrrole: conformational changes induced by variation of dopant and substrate surface. Synth Met 84:153–154. doi:10.1016/S0379-6779(97)80689-3
Bartlett PN, Cooper JM (1993) A review of the immobilization of enzymes in electropolymerized films. J Electroanal Chem 362:1–12
Bryaskova R, Pencheva D, Nikolov S, Kantardjiev T (2011) Synthesis and comparative study on the antimicrobial activity of hybrid materials based on silver nanoparticles (AgNps) stabilized by polyvinylpyrrolidone (PVP). J Chem Biol 4:185–191. doi:10.1007/s12154-011-0063-9
Devi DSP, Nair AB, Jabin T, Kutty SKN (2012) Mechanical, thermal, and microwave properties of conducting composites of polypyrrole/polypyrrole-coated short nylon fibers with acrylonitrile butadiene rubber. J Appl Polym Sci 126:1965–1976. doi:10.1002/App.36924
Dickert H, Machka K, Braveny I (1981) The uses and limitations of disc diffusion in the antibiotic sensitivity testing of bacteria. Infection 9:18–24. doi:10.1007/BF01640803
Foroughi J, Ghorbani SR, Peleckis G, Spinks GM, Wallace GG, Wang XL, Dou SX (2010) The mechanical and the electrical properties of conducting polypyrrole fibers. J Appl Phys. doi:10.1063/1.3425793
Foulds NC, Lowe CR (1986) Enzyme entrapment in electrically conducting polymers - immobilization of glucose-oxidase in polypyrrole and its application in amperometric glucose sensors. J Chem Soc Farad T 1(82):1259–1264. doi:10.1039/F19868201259
Gashti MP, Almasian A (2013) UV radiation induced flame retardant cellulose fiber by using polyvinylphosphonic acid/carbon nanotube composite coating. Compos Part B-Eng 45:282–289. doi:10.1016/j.compositesb.2012.07.052
Gashti MP, Ghehi ST, Arekhloo SV, Mirsmaeeli A, Kiumarsi A (2015) Electromagnetic shielding response of UV-induced polypyrrole/silver coated wool. Fiber Polym 16:585–592. doi:10.1007/s12221-015-0585-9
Gorjanc M, Bukosek V, Gorensek M, Mozetic M (2010) CF4 plasma and silver functionalized cotton. Text Res J 80:2204–2213. doi:10.1177/0040517510376268
Hock CW, Harris M (1940) Microscopic examination of cotton fibers in cuprammonium hydroxide solutions. J Res Natl Bur Stand 24:743–748
Iijima S (1991) Helical microtubules of graphitic carbon. Nature 354:56–58. doi:10.1038/354056a0
Jurewicz K, Delpeux S, Bertagna V, Beguin F, Frackowiak E (2001) Supercapacitors from nanotubes/polypyrrole composites. Chem Phys Lett 347:36–40. doi:10.1016/S0009-2614(01)01037-5
Kim JU, Jeong IS, Moon SI, Gu HB (2001) Electrochemical characteristics of LiMn2O4-polypyrrole composite cathode for lithium polymer batteries. J Power Sour 97–8:450–453. doi:10.1016/S0378-7753(01)00743-1
Makowski T, Kowalczyk D, Fortuniak W, Jeziorska D, Brzezinski S, Tracz A (2014) Superhydrophobic properties of cotton woven fabrics with conducting 3D networks of multiwall carbon nanotubes, MWCNTs. Cellulose 21:4659–4670. doi:10.1007/s10570-014-0422-0
Manik AC, Shailesh GP, Prasad RG, Ramesh NM, Shashwati S, Vikas BP (2011) Synthesis and characterization of polypyrrole (PPy). Thin Films Soft Nanosci Lett 1:6–10. doi:10.4236/snl.2011.11002
Patra S, Pandey AK, Sen D, Ramagiri SV, Bellare JR, Mazumder S, Goswami A (2014) Redox decomposition of silver citrate complex in nanoscale confinement: an unusual mechanism of formation and growth of silver nanoparticles. Langmuir 30:2460–2469. doi:10.1021/la4048787
Perelshtein I, Applerot G, Perkas N, Guibert G, Mikhailov S, Gedanken A (2008) Sonochemical coating of silver nanoparticles on textile fabrics (nylon, polyester and cotton) and their antibacterial activity. Nanotechnology. doi:10.1088/0957-4484/19/24/245705
Pieta P, Venukadasula GM, D’Souza F, Kutner W (2009) Preparation and selected properties of an improved composite of the electrophoretically deposited single-wall carbon nanotubes, electrochemically coated with a C-60-Pd and polybithiophene mixed polymer film. J Phys Chem C 113:14046–14058. doi:10.1021/Jp903891v
Ravindra S, Mohan YM, Reddy NN, Raju KM (2010) Fabrication of antibacterial cotton fibres loaded with silver nanoparticles via “Green Approach”. Colloid Surf A 367:31–40. doi:10.1016/j.colsurfa.2010.06.013
Sadeghnejad A, Aroujalian A, Raisi A, Fazel S (2014) Antibacterial nano silver coating on the surface of polyethylene films using corona discharge. Surf Coat Tech 245:1–8. doi:10.1016/j.surfcoat.2014.02.023
Sathishkumar M, Sneha K, Yun YS (2010) Immobilization of silver nanoparticles synthesized using Curcuma longa tuber powder and extract on cotton cloth for bactericidal activity. Bioresou Technol 101:7958–7965. doi:10.1016/j.biortech.2010.05.051
Schmid A, Sutton LR, Armes SP, Bain PS, Manfre G (2009) Synthesis and evaluation of polypyrrole-coated thermally-expandable microspheres: an improved approach to reversible adhesion. Soft Matter 5:407–412. doi:10.1039/B811246K
Sharma VK, Yngard RA, Lin Y (2009) Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interf 145:83–96. doi:10.1016/j.cis.2008.09.002
Shrivastava S, Bera T, Roy A, Singh G, Ramachandrarao P, Dash D (2007) Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology. doi:10.1088/0957-4484/18/22/225103
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interf Sci 275:177–182. doi:10.1016/j.jcis.2004.02.012
Treacy MMJ, Ebbesen TW, Gibson JM (1996) Exceptionally high young’s modulus observed for individual carbon nanotubes. Nature 381:678–680. doi:10.1038/381678a0
Vaisman L, Wagner HD, Marom G (2006) The role of surfactants in dispersion of carbon nanotubes. Adv Colloid Interf 128:37–46. doi:10.1016/j.cis.2006.11.007
Wang C, Zheng W, Yue Z, Too CO, Wallace GG (2011) Buckled, stretchable polypyrrole electrodes for battery. Appl Adv Mater 23:3580–3584. doi:10.1002/adma.201101067
Yuan X, Ding X-L, Wang C-Y, Ma Z-F (2013) Use of polypyrrole in catalysts for low temperature fuel cells. Energy Environ Sci 6:1105–1124. doi:10.1039/C3EE23520C
Zhang Q-w, Zhou X, Yang H-s (2004) Capacitance properties of composite electrodes prepared by electrochemical polymerization of pyrrole on carbon foam in aqueous solution. J Power Sour 125:141–147. doi:10.1016/S0378-7753(03)00818-8
Zou YJ, Xiang CL, Yang LN, Sun LX, Xu F, Cao Z (2008) A mediatorless microbial fuel cell using polypyrrole coated carbon nanotubes composite as anode material. Int J Hydrog Energy 33:4856–4862. doi:10.1016/j.ijhydene.2008.06.061
Acknowledgments
The authors would like to thank the financial support of this work from the National Science Centre (DEC-2011/03/B/ST8/06126).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Makowski, T., Kowalczyk, D., Fortuniak, W. et al. Electrochemical deposition of silver nanoparticle and polymerization of pyrrole on fabrics via conducting multiwall carbon nanotubes. Cellulose 22, 3063–3075 (2015). https://doi.org/10.1007/s10570-015-0725-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-015-0725-9