Abstract
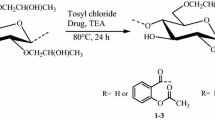
This article presents synthesis of novel macromolecular prodrugs of aceclofenac (an anti-inflammatory drug) onto hydroxypropylcellulose (HPC). The HPC-aceclofenac conjugates were prepared using an acylating agent 1,1′-carbonyldiimidazole (CDI) under homogenous reaction conditions. Aceclofenac was first activated by using CDI to form its N-acylimidazole. The N-acylimidazole of aceclofenac was then reacted with HPC polymer at 80 °C for 24 h. Highly pure prodrugs of aceclofenac were synthesized with a wide range of moderate to high degree of substitution (DS 0.41–2.12) as calculated by 1H NMR spectroscopy. The UV spectroscopic analysis has also revealed that the active drug aceclofenac was found in different conjugates from 28 to 67 mg/100 mg of HPC-aceclofenac conjugates which are in good agreement with DS calculated by 1H NMR spectroscopy. The gel permeation chromatography showed unimodal absorption that indicates no significant degradation in polymer chains during the reaction. The macromolecular prodrugs of aceclofenac were characterized using different spectroscopic and chromatographic techniques. The thermal analysis has revealed that HPC-aceclofenac conjugates (prodrugs) are 92 and 96 °C more stable than pure aceclofenac regarding their initial (Tdi) and maximum degradation temperatures (Tdm), respectively. The activation energy (Ea) and frequency factor (Z) of the degradation reactions were evaluated using Friedman, Broido and Chang methods. Degradation followed first order (n) kinetics. Transmission electron microscopy has revealed the formation of sponge like nano aggregates with population size distribution of around 80–150 nm.






Similar content being viewed by others
References
Amaral MH, Lobo JMS, Ferreira DC (2001) Effect of HPMC and hydrogenated castor oil on Naproxen release from sustained release tablets. AAPS Pharm Sci Tech 2: article 6
Duncan R, Gac-Breton S, Keane R, Musila R, Sat YN, Satchi R, Searle F (2001) Polymer-drug conjugates, PDEPT and PELT: basic principles for design and transfer from the laboratory to clinic. J Control Release 74:135–146
El-Samaligy M, El-Shakhs SA, Holail SM, Sabry NA (2003) Formulation and in-vivo investigation of riboflavin sustained release floating capsules. Egypt J Pharm Sci 44:73–85
Goodlett VW, Dougherty JT, Patton HW (1971) Characterization of cellulose acetates by nuclear magnetic resonance. J Polym Sci Part A 1: Pol Chem 9:155–161
Heinze T, Liebert TF, Pfeiffer KS, Hussain MA (2003) Unconventional cellulose esters: synthesis, characterization and structure-property relations. Cellulose 10:283–296
Hussain MA (2008) Unconventional synthesis and characterization of novel abietic acid esters of hydroxypropylcellulose as potential macromolecular prodrugs. J Polym Sci Part A: Pol Chem 46:747–752
Hussain MA, Heinze T (2008) Unconventional synthesis of pullulan abietates. Polym Bull 60:775–783
Hussain MA, Liebert TF, Heinze T (2004) Acylation of cellulose with N,N′-carbonyldiimidazole-activated acids in the novel solvent dimethyl sulfoxide/tetrabutylammonium fluoride. Macromol Rapid Comm 25:916–920
Hussain MA, Badshah M, Iqbal MS, Tahir MN, Tremel W, Bhosale SV, Sher M, Haseeb MT (2009) HPMC-salicylate conjugates as macromolecular prodrugs: design, characterization and nano-rods formation. J Polym Sci Part A: Pol Chem 47:4202–4208
Hussain MA, Abbas K, Sher M, Tahir MN, Tremel W, Iqbal MS, Amin M, Badshah M (2011) Macromolecular prodrugs of aspirin with HPMC: a nano particulate drug design, characterization, pharmacokinetic studies. Macromol Res 19:1296–1302
Ichikawa H, Onishi H, Takahata T, Machida Y, Nagai T (1993) Evaluation of the conjugate between N4-(4-carboxybutyryl)-1-beta-D-arabinofuranosylcytosine and chitosan as a macromolecular prodrug of 1-beta-D-arabinofuranosylcytosine. Drug Des Discov 10:343–353
Lee BJ, Ryu SG, Cui JH (1999) Controlled release of dual drug-loaded hydrooxyprpylmethylcellulose matrix tablet using drug-containing polymeric coatings. Int J Pharm 188:71–80
Lu X, Hu Z, Gao J (2000) Synthesis and light scattering study of hydroxypropylcellulose microgels. Macromolecules 33:8698–8702
Ochoa L, Igartua M, Hernandez RM, Gascon AR, Pedraz JL (2005) Preparation of sustained release hydrophilic matrices by melt granulation in a high-shear mixer. J Pharm Pharm Sci 8:132–140
Parejo C, Gallardo A, Roman JS (1998) Controlled release of NSAIDs bound to polyacrylic carrier systems. J Mater Sci Mater Med 9:803–809
Peng YS, Lin SC, Huang SJ, Wang YM, Lin YJ, Wang LF, Chen JS (2006) Chondroitin sulfate-based anti-Inflammatory macromolecular prodrugs. Eur J Pharm Sci 29:60–69
Rasheed A, Kumar CKA (2010) Design, synthesis, hydrolysis kinetics and phamacodynamic profiles of histidine and alanine conjugates of aceclofenac. Acta Pharm 60:99–109
Reddy KR, Mutalik S, Reddy S (2003) Once-daily sustained-release matrix tablets of nicorandil: formulation and in vitro evaluation. AAPS Pharm Sci Tech 4:480–488
Sharma R, Sharma CL (2008) Macromolecular drugs: novel strategy in target specific drug delivery. J Clin Diagnos Res 2:1020–1023
Shrivastava PK, Singh R, Shrivastava SK (2010) Polyamidoamine dendrimer and dextran conjugates: preparation, characterization, and in vitro and in vivo evaluation. Chem Pap 64:592–601
Singh AP, Ramadan WM, Dahiya R, Sarpal AS, Pathak K (2009) Product development studies of amino acid conjugate of aceclofenac. Curr Drug Del 6:208–216
Yamazaki N, Kojima S, Bovin NV, André S, Gabius S, Gabius HJ (2000) Endogenous lectins as targets for drug delivery. Adv Drug Deliv Rev 43:225–244
Acknowledgments
K. Abbas gratefully acknowledges financial support provided by the Higher Education Commission (HEC) of Pakistan under the scheme “HEC Indigenous 5000 fellowships Scheme”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hussain, M.A., Zarish, A., Abbas, K. et al. Hydroxypropylcellulose-aceclofenac conjugates: high covalent loading design, structure characterization, nano-assemblies and thermal kinetics. Cellulose 20, 717–725 (2013). https://doi.org/10.1007/s10570-012-9860-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-012-9860-8