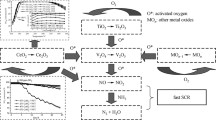
Abstract
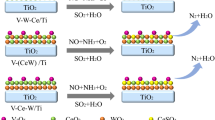
We investigate the effect of cerium and tungsten addition to optimize the deNOx activity of V2O5/TiO2 catalysts over a broad temperature range in the catalytic reduction of NOx with NH3 (NH3-SCR) with and without the presence of water. The catalysts were synthesized following co-impregnation of TiO2 with different loadings and varying content of V2O5, CeO2 and WO3 oxides as promoters. Based on surface and bulk characterization, we show that all catalysts undergo different structural changes depending on the chemical nature of the promoters. X-ray photoelectron spectra indicate a tendency for surface reduction after addition of CeO2, surface oxidation after addition of WO3, and after catalytic NH3-SCR. Promotion of V2O5/TiO2 catalysts with CeO2 and/or WO3 broadens the operation temperature window of the catalytic NH3-SCR reaction under both dry and wet conditions and improves the N2 selectivity at high temperatures. The thermal deactivation resistance of CeO2- and WO3-promoted catalysts improves with increasing amount of WO3. We tentatively relate this to suppression of the sintering of the active VOx component and increasing the amount of CeVO4. The latter, as a consequence of Ce-V interaction, detrimentally changes the surface composition of the catalysts and hides active V in the bulk structure inaccessible for reaction. Water slightly decreases the overall catalytic activity of SCR at low temperatures, while preventing the formation of N2O at elevated temperatures. Addition of CeO2 leads to a slight decrease in overall reducibility of the catalysts, while W causes an enhancement in quantitative H2 uptake. On the contrary, the sole addition of CeO2 leads to an enhancement of ammonia adsorption and the appearance of new acidic surface sites, which beneficially combine the reduced surface of the catalysts with an enhanced deNOx activity at low temperature.
Graphical Abstract













Similar content being viewed by others
References
Forzatti P (2001) Appl Catal A 222:221–236
Liu Z, Ihl Woo S (2006) Catal Rev 48:43–89
Organization WH (2003) Health aspects of air pollution with particulate matter, ozone and nitrogen dioxide: report on a WHO working group, Bonn, Germany 13–15 January 2003. WHO Regional Office for Europe, Copenhagen
Youn S, Jeong S, Kim DH (2014) Catal Today 232:185–191
Tarjomannejad A, Niaei A, Farzi A et al (2016) Catal Lett 146:1544–1551
Lietti L, Alemany J, Forzatti P et al (1996) Catal Today 29:143–148
Lietti L, Nova I, Forzatti P (2000) Top Catal 11:111–122
Yang W, Liu F, Xie L et al (2016) Ind Eng Chem Res 55:2677–2685
Zhao X, Yan Y, Mao L et al (2018) RSC Adv 8:31081–31093
Xiong S, Xiao X, Liao Y et al (2015) Ind Eng Chem Res 54:11011–11023
Han L, Cai S, Gao M et al (2019) Chem Rev 119:10916–10976
Fu M, Li C, Lu P et al (2014) Catal Sci Technol 4:14–25
Koebel M, Elsener M, Kleemann M (2000) Catal Today 59:335–345
Wu G, Li J, Fang Z et al (2015) Catal Commun 64:75–79
Marberger A, Elsener M, Ferri D et al (2015) ACS Catal 5:4180–4188
Casanova M, Llorca J, Sagar A et al (2015) Catal Today 241:159–168
Casanova M, Schermanz K, Llorca J et al (2012) Catal Today 184:227–236
Zhao X, Huang L, Li H et al (2015) Chin J Catal 36:1886–1899
Zhao X, Huang L, Namuangruk S et al (2016) Catal Sci Technol 6:5543–5553
Shan W, Liu F, He H et al (2012) Appl Catal, B 115:100–106
Liu Z, Zhang S, Li J et al (2014) Appl Catal B 158:11–19
Gu T, Liu Y, Weng X et al (2010) Catal Commun 12:310–313
Reddy BM, Khan A, Yamada Y et al (2002) J Phys Chem B 106:10964–10972
Gillot S, Tricot G, Vezin H et al (2018) Appl Catal B 234:318–328
Gillot S, Tricot G, Vezin H et al (2017) Appl Catal B 218:338–348
Chen L, Li J, Ge M (2009) J Phys Chem C 113:21177–21184
Liang Q, Li J, Yue T (2021) Environ Technol Innov 21:101209
Soleimanzadeh H, Niaei A, Salari D et al (2019) J Environ Manage 238:360–367
Watschinger M, Ploner K, Winkler D et al (2021) Rev Sci Instrum 92:024105
Mohammadi A, Farzi A, Thurner C et al (2022) Appl Catal, B 307:121160
Dunn JP, Stenger HG Jr, Wachs IE (1999) Catal Today 51:301–318
Bourikas K, Fountzoula C, Kordulis C (2004) Appl Catal B 52:145–153
Martınez-Huerta M, Coronado J, Fernández-Garcıa M et al (2004) J Catal 225:240–248
Huang Y, Tong Z-Q, Bing W et al (2008) J Fuel Chem Technol 36:616–620
Peng Y, Wang C, Li J (2014) Appl Catal B 144:538–546
Vuurman MA, Wachs IE, Hirt AM (1991) J Phys Chem 95:9928–9931
Horn M, Schwerdtfeger C, Meagher E (1972) Z Kristallogr Krist 136:273–281
Baur WH, Khan AA (1971) Acta Crystallogr B Struct Cryst Cryst Chem 27:2133–2139
Itoh T, Mori M, Inukai M et al (2015) J Phys Chem C 119:8447–8458
Mahapatra S, Madras G, Guru Row T (2007) Ind Eng Chem Res 46:1013–1017
Woodward P, Sleight A, Vogt T (1995) J Phys Chem Solids 56:1305–1315
Wachs IE, Roberts CA (2010) Chem Soc Rev 39:5002–5017
Reiche M, Buergi T, Baiker A et al (2000) Appl Catal A 198:155–169
Ross-Medgaarden EI, Wachs IE (2007) J Phys Chem C 111:15089–15099
Banares M, Wachs I (2002) J Raman Spectrosc 33:359–380
Wang C, Yang S, Chang H et al (2013) Chem Eng J 225:520–527
Peng Y, Li K, Li J (2013) Appl Catal B 140:483–492
Li P, Xin Y, Li Q et al (2012) Environ Sci Technol 46:9600–9605
Neri G, Pistone A, Milone C et al (2002) Appl Catal B 38:321–329
Choi E-Y, Nam I-S, Kim YG (1996) J Catal 161:597–604
Biesinger MC, Payne BP, Grosvenor AP et al (2011) Appl Surf Sci 257:2717–2730
Naumkin AV, Kraut-Vass A, Gaarenstroom SW, et al (2012) NIST standard reference database
Lee KJ, Kumar PA, Maqbool MS et al (2013) Appl Catal B 142:705–717
Xiaodong W, Zhichun S, Guo L et al (2011) J Rare Earths 29:64–68
Xu HY, Xu KW, Ma F et al (2018) RSC Adv 8:10064–10071
Kovács G, Baia L, Vulpoi A et al (2014) Appl Catal B 147:508–517
Boningari T, Koirala R, Smirniotis PG (2013) Appl Catal B 140:289–298
Lietti L, Ramis G, Berti F et al (1998) Catal Today 42:101–116
Topsoe N, Topsoe H, Dumesic J (1995) J Catal 151:226–240
Zhu M, Lai J-K, Tumuluri U et al (2017) J Am Chem Soc 139:15624–15627
Dong G-J, Yuan Z, Zhang Y-F (2014) J Fuel Chem Technol 42:1093–1101
Grünbacher M, Tarjomannejad A, Nezhad PDK et al (2019) J Catal 379:18–32
Kwon DW, Lee S, Kim J et al (2021) Catal Today 359:112–123
Sun C, Dong L, Yu W et al (2011) J Mol Catal A 346:29–38
Martín-Martín J, Gallastegi-Villa M, González-Marcos M et al (2021) Chem Eng J 417:129013
Sullivan JA, Keane O (2005) Appl Catal B 61:244–252
ToPsøE N-Y, Slabiak T, Clausen BS, et al. (1992) J Catal 134.
Turco M, Lisi L, Pirone R et al (1994) Appl Catal, B 3:133–149
Inomata Y, Kubota H, Hata S et al (2021) Nat Commun 12:1–11
Acknowledgements
The work was performed within the framework of the bilateral IMPULSE program, financed by funds of the OeAD and of the Ministry of Science, Research and Technology of the Islamic Republic of Iran and the special research platform “Materials and Nanoscience” at the University of Innsbruck.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohammadi, A., Praty, C., Farzi, A. et al. Influence of CeO2 and WO3 Addition to Impregnated V2O5/TiO2 Catalysts on the Selective Catalytic Reduction of NOx with NH3. Catal Lett 153, 2176–2195 (2023). https://doi.org/10.1007/s10562-022-04108-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-022-04108-x