Abstract
In this work, the Ni-X and Ni-CNTs-X (X = No, F, Cl, Br, or I) nano-composites are successfully synthesized via a simple hydrothermal synthesis method. The corresponding RuNi-X (or RuNi-CNTs-X) composites were prepared at room temperature by a simple galvanic replacement reaction. The morphologies of RuNi-CNTs were modulated by adding different halogen ions during the synthesis process. The RuNi-CNTs-X samples with various shapes showed distinct different catalytic performance in benzene hydrogenation. The change rule of the catalytic behaviors of the RuNi-CNTs-X catalysts was similar to that of RuNi-X. The order of the catalytic properties of RuNi-CNTs-X for benzene hydrogenation was RuNi-CNTs-F > RuNi-CNTs-No > RuNi-CNTs-Cl > RuNi-CNTs-Br > RuNi-CNTs-I.
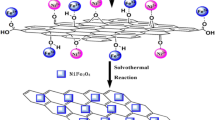
Graphic Abstract











Similar content being viewed by others
References
Wu B, Zheng N (2013) Surface and interface control of noble metal nanocrystals for catalytic and electrocatalytic applications. Nano Today 8:168–197
Wu L, Mendoza-Garcia A, Li Q, Sun S (2016) Organic phase syntheses of magnetic nanoparticles and their applications. Chem Rev 116:10473–10512
Chen S, Zhang B, Su D, Huang W (2015) Titania morphology-dependent gold–titania interaction, structure, and catalytic performance of gold/titania catalysts. ChemCatChem 7:3290–3298
Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ (1996) A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 382:607–609
Yuan T, Liu D, Pan Y, Pu X, Xia Y, Wang J, Xiong W (2018) Magnetic anchored CoPt bimetallic nanoparticles as selective hydrogenation catalyst for cinnamaldehyde. Catal Lett 149:851–859
Rong Z, Sun Z, Wang Y, Lv J, Wang Y (2014) Selective hydrogenation of cinnamaldehyde to cinnamyl alcohol over graphene supported Pt–Co bimetallic catalysts. Catal Lett 144:980–986
Yuan T, Liu D, Gu J, Xia Y, Pan Y, Xiong W (2019) The low dimensional Co-based nanorods as a novel platform for selective hydrogenation of cinnamaldehyde. Catal Lett 149:2906–2915
Polavarapu L, Mourdikoudis S, Pastoriza-Santos I, Pérez-Juste J (2015) Nanocrystal engineering of noble metals and metal chalcogenides: controlling the morphology, composition, and crystallinity. CrystEngComm 17:3727–3762
Nakanishi T, Masuda Y, Koumoto K (2004) Site-selective deposition of magnetite particulate thin films on patterned self-assembled monolayers. Chem Mater 16:3484–3488
Kim P, Epstein AK, Khan M, Zarzar LD, Lipomi DJ, Whitesides GM, Aizenberg J (2012) Structural transformation by electrodeposition on patterned substrates (STEPS): a new versatile nanofabrication method. Nano Lett 12:527–533
Shiju NR, Guliants VV (2007) Microwave-assisted hydrothermal synthesis of monophasic Mo-V-Te-Nb-O mixed oxide catalyst for the selective ammoxidation of propane. ChemPhysChem 8:1615–1617
Lucero J, Osuna C, Crawford JM, Carreon MA (2019) Microwave-assisted synthesis of porous organic cages CC3 and CC2. CrystEngComm 21:4534–4537
Cho S, Jung SH, Lee KH (2008) Morphology-controlled growth of ZnO nanostructures using microwave irradiation: from basic to complex structures. J Phys Chem C 112:12769–12776
Ummey R, Zannatul K, Nurul IM, Carl R, Takehiko Y (2018) A review on the recent advances in the reductions of carbon–carbon/oxygen multiple bonds including aromatic rings using raney Ni–Al alloy or al powder in the presence of noble metal catalysts in water. Top Catal 61:560–574
Hallaj R, Akhtari K, Salimi A, Soltanian S (2013) Controlling of morphology and electrocatalytic properties of cobalt oxide nanostructures prepared by potentiodynamic deposition method. Appl Surf Sci 276:512–520
Bakos I, Borbáth I, Vass Á, Pászti Z, Tompos A (2018) Design and investigation of molybdenum modified platinum surfaces for modeling of CO tolerant electrocatalysts. Top Catal 61:1385–1395
Fan Y, Lu HT, Liu JH, Yang CP, Jing QS, Zhang YX, Huang KJ (2011) Hydrothermal preparation and electrochemical sensing properties of TiO2–graphene nanocomposite. Colloid Surf B 83:78–82
Dükkancı M, Gündüz G, Yılmaz S, Prihod’Ko RV (2010) Heterogeneous fenton-like degradation of rhodamine 6G in water using CuFeZSM-5 zeolite catalyst prepared by hydrothermal synthesis. J Hazard Mater 181:343–350
Haddad K, Abokifa A, Kavadiya S, Chadha TS, Shetty P, Wang Y, Biswas P (2016) Growth of single crystal, oriented SnO2 nanocolumn arrays by aerosol chemical vapour deposition. CrystEngComm 18:7544–7553
Barreca D, Massignan C, Daolio S, Fabrizio M, Piccirillo C, Armelao L, Tondello E (2001) Composition and microstructure of cobalt oxide thin films obtained from a novel cobalt (II) precursor by chemical vapor deposition. Chem Mater 13:588–593
Jin BB, Zeng JH, Wang YF, Yang Q (2012) Uniform single-crystalline zinc oxide round nanodisks, a comprehensive study on the hydrothermal growth. Mater Lett 85:7–10
Kuriakose S, Satpati B, Mohapatra S (2014) Enhanced photocatalytic activity of Co doped ZnO nanodisks and nanorods prepared by a facile wet chemical method. Phys Chem Chem Phys 16:12741–12749
Li J, Zhang L, Zhu J, Liu Y, Hao W, Li B (2012) Controllable synthesis and magnetic investigation of ZnO: Co nanowires and nanotubes. Mater Lett 87:101–104
Varghese B, Teo CH, Zhu Y, Reddy MV, Chowdari BV, Wee ATS, Sow CH (2007) Co3O4 nanostructures with different morphologies and their field-emission properties. Adv Funct Mater 17:1932–1939
Ren ZF, Huang ZP, Xu JW, Wang JH, Bush P, Siegal MP, Provencio PN (1998) Synthesis of large arrays of well-aligned carbon nanotubes on glass. Science 282:1105–1107
Seo MH, Yuasa M, Kida T, Huh JS, Shimanoe K, Yamazoe N (2009) Gas sensing characteristics and porosity control of nanostructured films composed of TiO2 nanotubes. Sensor Actuat B-Chem 137:513–520
Kwak J, Kim CE, Min Y, Lee JH, Soon A, Jeong U (2015) The effect of Se doping on the growth of Te nanorods. CrystEngComm 17:5734–5743
Pan C, Zhang D, Shi L (2008) CTAB assisted hydrothermal synthesis, controlled conversion, and CO oxidation properties of CeO2 nanoplates, nanotubes, and nanorods. J Solid State Chem 181:1298–1306
Sau TK, Murphy CJ (2004) Room temperature, high-yield synthesis of multiple shapes of gold nanoparticles in aqueous solution. J Am Chem Soc 126:8648–8649
Hinotsu T, Jeyadevan B, Chinnasamy CN, Shinoda K, Tohji K (2004) Size and structure control of magnetic nanoparticles by using a modified polyol process. J Appl Phys 95:7477–7479
Mali SS, Betty CA, Bhosale PN, Devan RS, Ma YR, Kolekar SS, Patil PS (2012) Hydrothermal synthesis of rutile TiO2 nanoflowers using brønsted acidic ionic liquid: synthesis, characterization, and growth mechanism. CrystEngComm 14:1920–1924
Panwar A, Yadav KL (2015) A novel one-pot synthesis of hierarchical europium doped ZnO nanoflowers. Mater Lett 142:30–34
García B, Moreno J, Iglesias J, Melero JA, Morales G (2019) Transformation of glucose into sorbitol on raney nickel catalysts in the absence of molecular hydrogen: sugar disproportionation vs catalytic hydrogen transfer. Top Catal 62:570–578
Bai G, Dong H, Zhao Z, Wang Y, Chen Q, Qiu M (2013) Preparation of nanoscale Ni-B amorphous alloys and their application in the selective hydrogenation of cinnamic acid. J Nano Nanotechnol 13:5012–5016
Bai L, Fan J, Cao Y, Yuan F, Zuo A, Tang Q (2009) Shape-controlled synthesis of Ni particles via polyol reduction. J Cryst Growth 311:2474–2479
Chen G, Zhao Y, Lv D, Zhao T, Zhu Y, Sun Y (2013) Kinetically controlled synthesis of nickel tetrahedron nanocrystals for high performance catalytic hydrogenation. RSC Adv 3:5314–5317
Pan Y, Jia R, Zhao J, Liang J, Liu Y, Liu C (2014) Size-controlled synthesis of monodisperse nickel nanoparticles and investigation of their magnetic and catalytic properties. Appl Surf Sci 316:276–285
Wang C, Han X, Xu P, Wang J, Du Y, Wang X, Zhang T (2010) Controlled synthesis of hierarchical nickel and morphology-dependent electromagnetic properties. J Phys Chem C 114:3196–3203
Wang DP, Sun DB, Yu HY, Meng HM (2008) Morphology controllable synthesis of nickel nanopowders by chemical reduction process. J Cryst Growth 310:1195–1201
Zhu L, Jiang Y, Zheng J, Zhang N, Yu C, Li Y, Zhong CJ (2015) Ultrafine nanoparticle-supported Ru nanoclusters with ultrahigh catalytic activity. Small 11:4385–4393
Zhu L, Shan S, Petkov V, Hu W, Kroner A, Zheng J, Zhong CJ (2017) Ruthenium–nickel–nickel hydroxide nanoparticles for room temperature catalytic hydrogenation. J Mater Chem A 5:7869–7875
Zhu L, Zhang H, Ma N, Yu C, Ding N, Chen JL, Chen BH (2019) Tuning the interfaces in the ruthenium–nickel/carbon nanocatalysts for enhancing catalytic hydrogenation performance. J Catal 377:299–308
Rogatis LD, Montini T, Lorenzut B, Fornasiero P (2008) NixCuy/Al2O3 based catalysts for hydrogen production. Energy Environ Sci 1:501–509
Kim SI, Kang JH, Kim SW, Jang JH (2017) A new approach to high-performance flexible supercapacitors: mesoporous three-dimensional Ni-electrodes. Nano Energy 39:639–646
Cheng FY, Chen J, Gou XL (2006) MoS2–Ni nanocomposites as catalysts for hydrodesulfurization of thiophene and thiophene derivatives. Adv Mater 18:2561–2564
Li L, Shang Z, Xiao Z, Wang L, Liang X, Liu G (2019) Steam reforming of n-dodecane over mesoporous alumina supported nickel catalysts: effects of metal-support interaction on nickel catalysts. Int J Hydrogen Energy 44:6965–6977
Luo T, Qu L, Hou X, Liu X, Wang S, Wu Y (2017) Preparation of 3-dimensional flower-like NiFe2O4 with enhanced adsorptive performance for water contaminants. J Alloy Compd 727:484–490
Zhu L, Zheng T, Zheng J, Yu C, Zhang N, Zhou Q, Zhang W, Chen BH (2017) Shape control of nickel crystals and catalytic hydrogenation performance of ruthenium-on-Ni crystals. CrystEngComm 20:113–121
Zhu L, Cui J, Zhang H, Ruan L, Ma N, Zou L, Xiao Q (2019) Room-temperature morphology-controlled synthesis of nickel and catalytic properties of corresponding Ru/Ni catalysts. ChemCatChem 11:3109–3116
Sun Y, Li C, Zhang A (2016) Preparation of Ni/CNTs catalyst with high reducibility and their superior catalytic performance in benzene hydrogenation. Appl Catal A Gen 522:180–187
Nassr ABAA, Sinev I, Grünert W, Bron M (2013) PtNi supported on oxygen functionalized carbon nanotubes: in depth structural characterization and activity for methanol electrooxidation. Appl Catal B Environ 142:849–860
Li PP, Lang WZ, Xia K, Luan L, Yan X, Guo YJ (2016) The promotion effects of Ni on the properties of Cr/Al catalysts for propane dehydrogenation reaction. Appl Catal A Gen 522:172–179
Manukyan KV, Cross AJ, Yeghishyan AV, Rouvimov S, Miller JJ, Mukasyan AS, Wolf EE (2015) Highly stable Ni–Al2O3 catalyst prepared from a Ni–Al layered double hydroxide for ethanol decomposition toward hydrogen. Appl Catal A Gen 508:37–44
Mäkelä E, Lahti R, Jaatinen S, Romar H, Hu T, Puurunen RL, Karinen R (2018) Study of Ni, Pt, and Ru catalysts on wood-based activated carbon supports and their activity in furfural conversion to 2-methylfuran. ChemCatChem 10:3269–3283
Railsback JG, Johnston-Peck AC, Wang J, Tracy JB (2010) Size-dependent nanoscale kirkendall effect during the oxidation of nickel nanoparticles. ACS Nano 4:1913–1920
Le TA, Kang JK, Park ED (2018) CO and CO2 methanation over Ni/SiC and Ni/SiO2 catalysts. Top Catal 61:1537–1544
Choi JH, Lee C, Lee DB (2008) Oxidation behavior of bulk amorphous Ni57Ti18Zr20Si3Sn2 coatings between 473 and 973 K in air. J Alloy Compd 449:384–388
Gou W, Li J, Gao W, Xia Z, Zhang S, Ma Y (2019) Downshifted d-band center of Ru/MWCNTs by turbostratic carbon nitride for efficient and robust hydrogen evolution in alkali. ChemCatChem 11:1970–1976
Wang X, Lan G, Liu H, Zhu Y, Li Y (2018) Effect of acidity and ruthenium species on catalytic performance of ruthenium catalysts for acetylene hydrochlorination. Catal Sci Technol 8:6143–6149
Hossain MN, Chen S, Chen A (2018) Fabrication and electrochemical study of ruthenium–ruthenium oxide/activated carbon nanocomposites for enhanced energy storage. J Alloys Compd 751:138–147
Zhou H, Huang Y, Cheng Y, Wang L, Li X (2017) Hydrogenation of methyl methacrylate under mild conditions using biosynthesis Ru catalyst. J Ind Eng Chem 47:221–227
Acknowledgements
This research was supported by the funding supports of the National Natural Science Foundation of China (Grant No. 21763011), Natural Science Foundation of Jiangxi Province for Distinguished Young Scholars (Grant No. 20192BCB23015), Natural Science Foundation of Jiangxi Province of China (Grant No. 20202BAB203005), Youth Jinggang Scholars Program in Jiangxi Province ([2019]57). China Postdoctoral Science Foundation (Grant No. 2018M642597), Foundation of State Key Laboratory of Coal Conversion (Grant No. J20-21-609), Research Foundation of the Education Bureau of Jiangxi Province of China (GJJ190429), Postdoctoral Science Foundation of Jiangxi Province of China, Program of Qingjiang Excellent Young Talents, Jiangxi University of Science and Technology (JXUSTQJBJ2019002), Program of Technology Innovation Talents of Ganzhou.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pei, A., Ruan, L., Zeng, P. et al. Controlled Synthesis of RuNi-CNTs Nano-Composites and Their Catalytic Performance in Benzene Hydrogenation. Catal Lett 151, 773–786 (2021). https://doi.org/10.1007/s10562-020-03341-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03341-6