Abstract
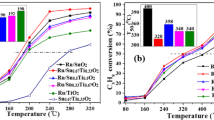
This study evaluates: (1) Ru-containing catalysts for low temperature hydrogenation of acetic acid in aqueous medium to simulate hydrogenating bio-derived oils, and (2) the development of the role of ruthenium–titania (Ru–TiO2) catalytic interaction for this system. A series of 9 catalysts was screened, and a comparison of selectivity versus conversion indicated that selectivity was a function of conversion, with ethanol being the predominant product at low conversion, and light gases being favored at higher conversion by secondary reactions. Exponential trend curves for ethanol (decay) and methane (increase) with conversion were fitted. On a per gram catalyst basis, the most active catalyst under study in the temperature range of 120° through 220 °C with a hydrogen partial pressure of 1000 psi (7.0 MPa) was 5 % Ru on carbon; however, its selectivity for the conversion to ethanol was exceptionally low (5 % selectivity at ~70 % conversion and 180 °C) with the primary products being ethane and methane. This catalyst formulation displayed a negative deviation from the trend curve for ethanol and a higher methane selectivity deviation. On the other hand, a catalyst of Ru prepared by atomic layer deposition (i.e., Ru(ALD)/Ti(ALD)/Nb/Si) was also highly active (~55 % conversion at 180 °C) but displayed a significant positive deviation from the trend curve (~40 % selectivity). These results combined with those of EXAFS suggest that the interface between the deposited Ru and the titania support may be responsible for the increase in selectivity to ethanol. In general, the catalysts prepared by ALD were more active on a per gram catalyst basis than the catalysts prepared by standard aqueous impregnation. Samples of catalyst that were observed using transmission electron microscopy confirmed that the Ru was well dispersed in that no Ru nanoparticle morphology was observed within the resolving power of the JEOL JEM-3010 TEM instrument. Regarding the nanostructure of the support, TEM measurements revealed that the ALD method resulted in support domain sizes that were significantly smaller (<5 nm) as compared to a commercial titania (>10 nm), promoting defect formation. EXAFS characterization indicated that the best ALD catalyst (i.e., Ru(ALD)/Ti(ALD)/Nb/Si) had higher dispersion (i.e., smaller nanoparticles and thus greater metal-support interface) than the reference catalysts prepared by aqueous impregnation. A Ru-Osupport contribution was required in order to obtain an acceptable EXAFS fit (‘the long metal–oxygen bond’). Increases in Ru–Ru coordination along with decreases in Ru-Osupport coordination were observed for longer catalyst aging times and higher treatment temperatures. In summary, the selectivity to ethanol during hydrogenation of acetic acid was promoted by preparing finely dispersed Ru particles in close interaction with nanoscale titania domains.
Graphical Abstract
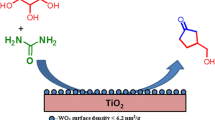
The hydrogenation of the bio-model compound, acetic acid, over Ru-deposited catalyst leads to ethanol and methane/ethane. Based on XANES/EXAFS results, the reaction on finely dispersed Ru interacting with titania leads to the preferred product, ethanol, while larger Ru metal particles in the absence of contact between Ru and titania leads towards the formation of light hydrocarbon gases.
















Similar content being viewed by others
References
Elliott DC (2007) Energy Fuels 21:1792
Wang H, Male J, Wang Y (2013) ACS Catal 3:1047
Alonso DM, Wettstein SG, Dumesic JA (2012) Chem Soc Rev 41:8075
Boonyasuwat S, Omotoso T, Resasco DE, Crossley SP (2013) Catal Lett 143:783
Elliott DC, Hart TR (2009) Energy Fuels 23:631
Wildschut J, Melian-Cabrera I, Heeres HJ (2010) Appl Catal B 99:298
Wildschut J, Mahfud FH, Venderbosch RH, Heeres HJ (2009) Ind Eng Chem Res 48:10324
Lee J, Kim YT, Huber GW (2014) Green Chem 16:708
Wan S, Pham T, Zhang S, Lobban L, Resasco DE, Mallinson R (2013) AIChE J 59(7):2275
Pham T, Shi D, Resasco DE (2014) Top Catal 57:706
Boullosa-Eiras S, Lødeng R, Bergem H, Stöcker M, Hannevold L, Blekkan EA (2014) Catal Today 223:44
de Souza PM, Rabelo-Neto RC, Borges LEP, Jacobs G, Davis BH, Sooknoi T, Resasco DE, Noronha FB (2015) ACS Catal 5:1318
Elam JW, Libera JA, Pellin MJ (2007) Appl Phys Lett 91:243105
Baumann TF, Biener J, Wang YM, Kucheyev SO, Nelson EJ, Satcher JH, Elam JW, Pellin MJ, Hamza AV (2006) Chem Mater 18:6106
Lu J, Low KB, Lei Y, Libera JA, Nicholls A, Stair PC, Elam JW (2014) Nat Commun 5:3264
Lu J, Fu B, Kung MC, Xiao G, Elam JW, Kung HH, Stair PC (2012) Science 335:1205
Pagán-Torres JY, Gallo JMR, Wang D, Pham NN, Libera JA, Marshall CL, Elam JW, Datye AK, Dumesic JA (2011) ACS Catal 1:1234
Elam JW, Groner MD, George SM (2002) Rev Sci Instrum 73:2981
Jacoby M (2001) Chem Eng News 79:33
Ressler T (1998) J Synchrotron Radiat 5:118
Ravel B (2001) J Synch Rad 8:314
Rehr JJ, Zabinsky SI, Albers RC (1992) Phys Rev Lett 69:3397
Newville M, Ravel B, Haskel D, Stern EA, Yacoby Y (2005) Physica B 208/209:154
Gursahani KI, Alcala R, Cortright RD, Dumesic JA (2001) Appl Catal A 222:369
Joshi N, Lawal A (2002) Chem Eng Sci 84:761
Altamira (Instruments) Notes (Vol. 1.2)
Lu J, Elam JW, Stair PC (2013) Acc Chem Res 46:1806
Panagiotopoulou P, Kondarides DI (2004) J Catal 225:327
Acknowledgments
This material is based upon work supported in part by the Institute for Atom-efficient Chemical Transformations (IACT), an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences. Additional support was provided by the Biomass Program of the Energy Efficiency and Renewable Energy Office of the U.S. Department of Energy under Contract No. DE-AC02-06CH11357. Experiments conducted at UK-CAER were supported by the Commonwealth of Kentucky. The use of the APS was supported by the U.S. DOE, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. MRCAT operations are supported by the DOE and the MRCAT member institutions. JAL and JWE are grateful to Tosoh, Inc. for supplying the ALD Ru precursor.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hedlund, J.K., Cronauer, D.C., Jacobs, G. et al. Titania Supported Ru Nanoclusters as Catalysts for Hydrodeoxygenation of Pyrolysis Oils. Catal Lett 146, 525–539 (2016). https://doi.org/10.1007/s10562-015-1669-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-015-1669-2