Abstract
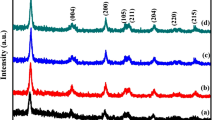
Transition metal doping into the TiO2 lattice can expand the response of these metal oxide nano particles to the visible region. In view of this, Mo6+ ion is doped into the TiO2 lattice in order to understand the mechanism of its photo response. The prepared photocatalysts were characterized by X-ray diffraction, UV–Visible absorption spectroscopy, UV–Visible diffuse reflectance spectroscopy, SEM, EDX, FTIR and BET specific surface area techniques. The characterization results have confirmed the incorporation of metal ions into TiO2 lattice. XRD analysis shows no change in crystal structure except a slight variation in crystallite size and elongation along the c-axis with increase in the concentration of the dopant. Diffuse reflectance measurements showed a shift in the band edge position to longer wavelengths and an extension of the absorption to the visible region. The photo degradation efficiencies of these catalysts were studied with Tebuconazole pesticide as model pollutant. Under UV light, undoped catalyst showed higher activity than doped catalyst. But in the case of visible light irradiation Mo doped TiO2 with intermediate dopant concentration of 0.06 atom % had the highest photocatalytic reactivity. This may be due to the narrowing of band gap so that it could effectively absorb the light of longer wavelength. The degradation path way was followed by UV–Visible spectroscopy.











Similar content being viewed by others
References
Pelizzetti E, Serpone N (eds) (1986) Homogeneous and heterogeneous photocatalysis. Reidel, Dordrecht
Schiavello M (1988) Photocatalysis and environment. Trends and applications. Kluwer Academic Publishers, Dordrecht
Fujishima A, Rao TN, Tryk DA (2000) J Photochem Photobiol C: Photochem Rev 1:1–21
Augugliaro V, Loddo V, Marchi G, Palmisano L, lopezmunoz MJ (1997) J Catal 166:272
Guha S, Ghosh K, Keeth JG, Ogale SB, Shinde SR, Simpson JR, Drew HD, Venkatesan T (2003) Appl Phys Lett 83:3296
Weng HM, Yang XP, Dong JM, Mizuseki H, Kawasaki M, Kawazoe Y (2004) Phys Rev B 69:125219
Matsumoto Y, Murakami M, Shono T, Hasegawa T, Fukumura T, Kawasaki M, Ahmet P, Chikyow T, Koshihara S, Koinuma H (2001) Science 291:854
Katagi T (2004) Rev Environ Contam Toxicol 182:1–195
Gomathi Devi L, Krishnaiah GM (1999) J Photochem Photobiol A: Chem 121:141–145
Azaroff LV (1977) Introduction to solids, TMH edn. McGraw-Hill Inc., New York
Prasad K, Bally Philippe AR, Schmid E, Lévy F, Benoit J, Barthou C, Benalloul P (1997) J Appl Phys 36:5696–5702
Lee JD (1996) Concise inorganic chemistry, 5th edn. Chapmann and Hall Publication, London
Kutty TRN, Gomathi Devi L (1985) Mater Res Bull 20:793
Fuller MP, Griffiths PR (1978) Anal Chem 5013:1906–1910
Cardona M, Harbeke G (1964) Phys Rev 137A:1467
Fuller MP, Griffiths PR (1978) Anal Chem 50(13):1906–1910
Kröger FA, Vink HJ (1956) Solid state physics. Academic Press, New York
Bernasik A, Radecka M, Rekas M, Sloma M (1993) Appl Surf Sci 65:240
Mizushima K, Tanaka M, Asai A, Ilda S, Goodenough JB (1979) J Phys Chem Solids 40:1129
Suda Y, Kawasaki H, Ueda T, Ohshima T (2004) Thin Solid Films 453–454:162
Wawrzyniak B, Morawski AW, Tryba B (2006) Int J Photoener 2006:1–8
Yoko XT, Kamiya K, Tanaka K (1990) J Mater Sci 25:3922
Zhang J, Boyd I, Sullivan BJO, Hurley PK, Kelly PV, Senateur JP (2002) J Non-Cryst Solid 303:134
Yamakata A, Aki Ishibashi T, Onishi H (2002) Bull Chem Soc Jpn 75:1019
Jackson P, Parfitt GD (1971) Trans Faraday Soc 67:2469
Maruska HP, Ghosh AK (1979) Solar Energy Mater 1:237
Acknowledgements
Financial support by UGC-Major Research Project (2007–2010), UGC-DRS, and UGC-FIST is greatly acknowledged. One of the author B. Narasimha Murthy wishes to acknowledge CMR Institute of Technology, Bangalore for its support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gomathi Devi, L., Narasimha Murthy, B. Characterization of Mo Doped TiO2 and its Enhanced Photo Catalytic Activity Under Visible Light. Catal Lett 125, 320–330 (2008). https://doi.org/10.1007/s10562-008-9568-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-008-9568-4