Abstract
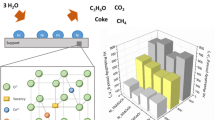
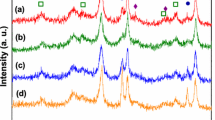
Steam reforming (SR) and oxidative steam reforming (OSR) of ethanol were investigated over undoped and Cu, Co and Ca doped Ni/CeO2–ZrO2 catalyst in the temperature range of 400–650 °C. The nickel loading was kept fixed at 30 wt.% and the loading of Cu and Co was varied from 2 to 10 wt% whereas the Ca loading was varied from 5 to 15 wt.%. The catalysts were characterized by various techniques, such as surface area, temperature programmed reduction, X-Ray diffraction and H2 chemisorption. For Cu and Co doped catalyst, CuO and Co3O4 phases were detected at high loading whereas for Ca doped catalyst, no separate phase of CaO was found. The reducibility and the metal support interactions were different for doped catalysts and varied with the amount and nature of dopants. The hydrogen uptake, nickel dispersion and nickel surface area was reduced with the metal loading and for the Co loaded catalysts the dispersion of Ni and nickel surface area was very low. For Cu and Ca doped catalysts, the activity was increased significantly and the main products were H2, CO, CH4 and CO2. However, the Co doped catalysts showed poor activity and a relatively large amount of C2H4, C2H6, CH3CHO and CH3COCH3 were obtained. For SR, the maximum enhancement in catalytic activity was obtained with in the order of NCu5. For Cu–Ni catalysts, CH3CHO decomposition and reforming reaction was faster than ethanol dehydrogenation reaction. Addition of Cu and Ca enhanced the water gas shift (WGS) and acetaldehyde reforming reactions, as a result the selectivity to CO2 and H2 were increased and the selectivity to CH3CHO was reduced significantly. The maximum hydrogen selectivity was obtained for Catalyst N (93.4%) at 650 °C whereas nearly the same selectivity to hydrogen (89%) was obtained for NCa10 catalyst at 550 °C. In OSR, the catalytic activity was in the order N > NCu5 > NCa15 > NCo5. In the presence of oxygen, oxidation of ethanol was appreciable together with ethanol dehydrogenation. For SR reaction, the highest hydrogen yield was obtained on the undoped catalyst at 600 °C. However, with calcium doping the hydrogen yields are higher than the undoped catalyst in the temperature range of 400–550 °C.









Similar content being viewed by others
References
Marino F., Boveri M., Baronetti G., Laborde M. (2001) Int. J. Hydrogen Energy 26:665
Breen J.P., Burch R., Coleman H.M. (2002) Appl. Catal. B: Environ. 39:65
Liguras D.K., Kondarides D.I., Verykios X.E. (2003) Appl.Catal. B: Environ. 43:345
Goula M.A., Kontou S.K., Tasiakaras P.E. (2004) Appl. Catal. B: Environ. 49:135
Fatsikostas A.N., Kondarides D.I., Verykios X.E. (2002) Catal. Today 75:145
Vaidya P.D., Rodrigues A.E. (2006) Chem. Eng. J. 117:39
Haryanto A., Fernando S., Murali N., Adhikari S. (2005) Energy Fuels 19:2098
Yang Y., Ma J., Wu F. (2006) Int. J. Hydrogen Energy 31(7):877
Sun J., Qiu X.P., Wu F., Zhu W.T. (2005) Int. J. Hydrogen Energy 30:437
Frusteri F., Freni S., Chiodo V., Spadaro L., Di Blasi O., Bonura G., Cavallaro S. (2004) Appl. Catal. A: Gen. 270:1
Frusteri F., Freni S., Chiodo V., Donato S., Bonura G. and Cavallaro S. (2006) Int. J Hydrogen Energy 31:2193
Fatsikostas A.N., Verykios X.E. (2004) J. Catal. 225:439
Aupretre F., Descorme C., Duprez D. (2002) Catal. Commun. 3:263
P. Biswas and D. Kunzru, Int. J. Hydrogen Energy (2006), doi: 10.1016/j.ijhydene.2006.09.031
Sayle T.X.T., Parker S.C., Catlow C.R.A. (2004) Surf. Sci. 316:329
Leitenburg C., Trovarelli A., Llorca J., Cavani F., Bini G. (1996) Appl. Catal. A: Gen. 139:161
Marino F.J., Cerrella E.G., Duhalde S., Jobbagy M., Laborde M.A. (1998) Int. J. Hydrogen Energy 23(12):1095
Marino F., Boveri M., Baronetti G., Laborde M. (2001) Int. J. Hydrogen Energy 26:665
Marino F., Baronetti G., Jobbagy M., Laborde M. (2003) Appl. Catal. A: Gen. 238:41
Marino F., Boveri M., Baronetti G., Laborde M. (2004) Int. J. Hydrogen Energy 29:67
Lisboa J.S., Santos D.C.R.M., Passos F.B., Noronha F.B. (2005) Catal. Today 101:15
Youn M.H., Seo J.G., Kim P., Kim J.J., Lee H.I., Song I.K. (2006) J. Power Sources 162(2):1270
Velu S., Suzuki K., Vijayaraj M., Barman S., Gopinath C.S. (2005) Appl. Catal. B: Environ 55:287
Fierro V., Akdim O., Provendier H., Mirodatos C. (2005) J. Power Sources 145:659
Lee J.H., Lee E.G., Joo O.S., Jung K.D. (2004) Appl. Catal. A: Gen 269:1
Huang T.J., Yu T.C., Jhao S.Y. (2006) Ind. Eng. Chem. Res. 45:150
Hu X., Lu G. (2007) J. Mol. Catal. A: Chem. 261:43
Cavallaro S., Chiodo V., Vita A., Freni S. (2003) J. Power Sources 123:10
Klouz V., Fierro V., Denton P., Katz H., Lisse J.P., Bouvot-Mauduit S., Mirodatos C. (2002) J. Power sources 105:26
Vesselli E., Comelli G., Rosei R., Freni S., Frusteri F., Cavallaro S. (2005) Appl. Catal. A: Gen 281:139
Arias A.M., Garcia M.F., Ballesteros V., Salamanca L.N., Conesa J.C., Otero C., Soria J. (1999) Langmuir 15:4796
Batista M.S., Santos R.K.S., Assaf E.M., Assaf J.M., Ticianelli E.A. (2003) J. Power Sources 124:99
Araya P., Guerrero S., Robertson J., Gracia F.J. (2005) Appl. Catal. a: Gen. 283:217
Papavasiliou J., Avgouropoulos G., Ioannides T. (2007) Appl. Catal B: Environ. 69:226
Zhang B., Tang X., Li Y., Cai W., Xu Y., Shen W. (2006) Catal. Commun. 7:367
Batista M.S., Assaf E.M., Assaf J.M., Ticianelli E.A. (2006) Int. J. Hydrogen Energy 31:1204
Sinfelt J.H., Yates D.J.C. (1967) J. Catal. 8:82
Comas J., Marino F., Laborde M., Amadeo N. (2004) Chem. Eng. J. 98:61
Huang T.J., Jhao S.Y. (2006) Appl. Catal. A: Gen. 302:325
Huang T.J., Yu T.C., Jhao S.Y. (2006) Ind. Eng. Chem. Res. 45:150
Patel S., Pant K.K. (2006) J. Power Sources 159:139
Yao C.Z., Wang L.C., Liu Y.M., Wu G.S., Cao Y., Dai W.L., He H.Y., Fan K.N. (2006) Appl. Catal. A: Gen. 297:151
Suetsuna T., Suenaga S., Fukasawa T. (2004) Appl. Catal. A: Gen. 276:275
Zhang Z., Baerns M. (1991) Appl. Catal. 75:299
Xu G., Murakami T., Suda T., Matsuzawa Y., Tani H., Mito Y., Ashizawa M. (2006) AIChE 52(10):3555
Chang J.S., Hong D.Y., Li X., Park S.E. (2006) Catal. Today 115:186
Freni S., Cavallaro S., Mondello N., Spadaro L., Frusteri F. (2003) Catal. Commun. 4:259
Llorca J., Homs N., Sales J., Piscina P.R. (2002) J. Catal. 209:306
Llorca J., Piscina P.R., Dalmon J.A., Sales J., Homs N. (2003) Appl. Catal. B: Environ. 43:355
Cavallaro S., Chiodo V., Freni S., Mondello N., Frusteri F. (2003) Appl. Catal. A: Gen 249:119
Cordi E.M., O’Neill P.J., Falconer J.L. (1997) Appl. Catal. B: Environ. 14(1–2):23
Laosiripojana N., Assabumrungrat S. (2006) Appl. Catal. B: Environ. 66:29
Hu Y.H., Ruckenstein E. (1996) Catal. Lett. 42:145
Nichio N., Casella M., Ferretti O., González M., Nicot C., Moraweck B., Frety R. (1996) Catal. Lett. 42:65
Dias J.A.C., Assaf J.M. (2003) Catal. Today 85:59
Frusteri F., Freni S., Chiodo V., Spadaro L., Di Blasi O., Bonura G., Cavallaro S. (2004) Appl. Catal. A: Gen. 270:1
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Biswas, P., Kunzru, D. Steam reforming of ethanol on Ni–CeO2–ZrO2 catalysts: Effect of doping with copper, cobalt and calcium. Catal Lett 118, 36–49 (2007). https://doi.org/10.1007/s10562-007-9133-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-007-9133-6