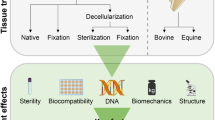
Abstract
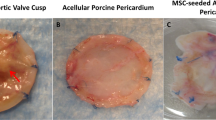
Glutaraldehyde (GA)-fixed bovine pericardial patches remain the cardiovascular industry standard despite reports of degradation, thickening, inflammation, calcification and lack of tissue remodelling. Decellularization provides the opportunity to attenuate some of these immune-mediated processes. This study compared the mechanical and morphological integrity of bovine pericardium that is GA-fixated (Glycar® patches) or decellularized (BPS), using a proprietary protocol, following implantation in an ovine model. The impact of the processing methods on tissue strength and morphology was assessed prior to implantation. Pericardial patches were then implanted in the descending aorta and main pulmonary artery of juvenile sheep (n = 6 per group) for 180 days, and clinically evaluated using echocardiography. At explanation, patches were evaluated for strength, calcification and biological interaction. Histology demonstrated a wave-like appearance of well-separated collagen fibers for BPS scaffolds that provided pore sizes adequate to promote fibroblast infiltration. The collagen of the Glycar® patches showed loss of collagen fiber integrity, making the collagen densely compacted, contributing to insignificant recipient cell infiltration. The clinical performance of both groups was excellent, and echocardiography confirmed the absence of aneurysm formation, calcification and degeneration. Explanted Glycar® patches demonstrated cells in abundance within the fibrous encapsulation that separated the implant from the host tissue. More importantly, the fibrous encapsulation also contributed to patch thickening of both the explanted aorta and pulmonary patches. The decellularized pericardial scaffolds demonstrated recellularization, resistance to calcification, re-endothelialization and adequate strength after 180-day implantation. The proprietary decellularization protocol produced pericardial scaffolds that could be considered as an alternative to GA-fixed pericardial patches.








Similar content being viewed by others
Availability of data and material
The manuscript has no associated data.
References
Badylak SF, Gilbert TW (2008) Immune response to biologic scaffold materials. Semin Immunol 20:109–116. https://doi.org/10.1016/j.smim.2007.11.003
Bancroft JD, Stevens A (1991) Theory and practice of histological techniques. Churchill Livingstone, Edinburg, p 0443035598
Baucia JR, Leal Neto RM, Rogero JR, Do Nascimento N (2006) Anti-calcification treatment of glutaraldehyde fixed bovine pericardium: comparison and evaluation of possible synergic effects. Braz J Cardiovasc Surg 21:8. https://doi.org/10.1590/S0102-76382006000200011
Bester D, Smit FE, Van Den Heever JJ, Botes L ,Dohmen PMCE (2017) Detoxification and stabilization of implantable or transplantable biological material. EU patent application
Bielli A, Bernardini R, Varvaras D, Rossi P, Di Blasi G, Petrella G, Buonomo OC, Mattei M, Orlandi A (2019) Corrigendum to “Characterization of a new decellularized bovine pericardial biological mesh: Structural and mechanical properties” [J. Mech. Behav. Biomed. Mater. 78 (2018) 420–426]. J Mech Behav Biomed Mater 94:317–318. https://doi.org/10.1016/j.jmbbm.2019.03.007
Canty EG, Kadler KE (2005) Procollagen trafficking, processing and fibrillogenesis. J Cell Sci 118:1341–1353. https://doi.org/10.1242/jcs.01731
Cohen S, Magal S, Yakov I, Sirabella E, Bitman A, Groisman G, Lotan C (2018) Tissue processing techniques for fabrication of covered stents for small-diameter vascular intervention. Acta Biomater 65:248–258. https://doi.org/10.1016/j.actbio.2017.10.044
Coito AJ, Kupiec-Weglinski JW (1996) Extracellular matrix proteins: bystanders or active participants in the allograft rejection cascade? Ann Transpl 1:14–18
Collatusso C, Roderjan JG, Vieira ED, Myague NI, Noronha L, Costa FD (2011) Decellularization as an anticalcification method in stentless bovine pericardium valve prosthesis: a study in sheep. Rev Bras Cir Cardiovasc 26:419–426. https://doi.org/10.5935/1678-9741.20110017
Courtman DW, Pereira CA, Kashef V, Mccomb D, Lee JM, Wilson GJ (1994) Development of a pericardial acellular matrix biomaterial: biochemical and mechanical effects of cell extraction. J Biomed Mater Res 28:655–666. https://doi.org/10.1002/jbm.820280602
Crapo PM, Gilbert TW, Badylak SF (2011) An overview of tissue and whole organ decellularization processes. Biomaterials 32:3233–3243. https://doi.org/10.1016/j.biomaterials.2011.01.057
Cross PC, Mercer KL (1993) Cell and tissue ultrastructure—a functional perspective. WH Freeman, New York
Dahm M, Lyman WD, Schwell AB, Factor SM, Frater RW (1990) Immunogenicity of glutaraldehyde-tanned bovine pericardium. J Thorac Cardiovasc Surg 99:1082–1090
Delgado LM, Bayon Y, Pandit A, Zeugolis DI (2015) To cross-link or not to cross-link? Cross-linking associated foreign body response of collagen-based devices. Tissue Eng Part B Rev 21:298–313. https://doi.org/10.1089/ten.TEB.2014.0290
Gilbert TW, Sellaro TL, Badylak SF (2006) Decellularization of tissues and organs. Biomaterials 27:3675–3683. https://doi.org/10.1016/j.biomaterials.2006.02.014
Huang-Lee LLH, Cheung DT, Nimni ME (1990) Biochemical changes and cytotoxicity associated with the degradation of polymeric glutaraldehyde derived crosslinks. J Biomed Mater Res 24:1185–1201. https://doi.org/10.1002/jbm.820240905
Hülsmann J, Grün K, El Amouri S, Barth M, Hornung K, Holzfuß C, Lichtenberg A, Akhyari P (2012) Transplantation material bovine pericardium: biomechanical and immunogenic characteristics after decellularization vs. glutaraldehyde-fixing. Xenotransplantation 19:286–297. https://doi.org/10.1111/j.1399-3089.2012.00719.x
Ionescu MI, Pakrashi BC, Holden MP, Mary DA, Wooler GH (1972) Results of aortic valve replacement with frame-supported fascia lata and pericardial grafts. J Thorac Cardiovasc Surg 64:340–353
Ionescu MI, Tandon AP, Mary DA, Abid A (1977) Heart valve replacement with the Ionescu-Shiley pericardial xenograft. J Thorac Cardiovasc Surg 73:31–42
Iop L, Palmosi T, Dal Sasso E, Gerosa G (2018) Bioengineered tissue solutions for repair, correction and reconstruction in cardiovascular surgery. J Thorac Dis 10:S2390–S2411. https://doi.org/10.21037/jtd.2018.04.27
Jayakrishnan A, Jameela SR (1996) Glutaraldehyde as a fixative in bioprostheses and drug delivery matrices. Biomaterials 17:471–484. https://doi.org/10.1016/0142-9612(96)82721-9
Ketchedjian A, Jones AL, Krueger P, Robinson E, Crouch K, Wolfinbarger L, Hopkins R (2005) Recellularization of decellularized allograft scaffolds in ovine great vessel reconstructions. Ann Thorac Surg 79:888–896; discussion 896. https://doi.org/10.1016/j.athoracsur.2004.09.033
Laker L, Dohmen PM, Smit FE (2020) Synergy in a detergent combination results in superior decellularized bovine pericardial extracellular matrix scaffolds. J Biomed Mater Res B Appl Biomater. https://doi.org/10.1002/jbm.b.34588
Li N, Li Y, Gong D, Xia C, Liu X, Xu Z (2018) Efficient decellularization for bovine pericardium with extracellular matrix preservation and good biocompatibility. Interact Cardiovasc Thorac Surg 26:768–776. https://doi.org/10.1093/icvts/ivx416
Mallis P, Michalopoulos E, Dimitriou C, Kostomitsopoulos N, Stavropoulos-Giokas C (2017) Histological and biomechanical characterization of decellularized porcine pericardium as a potential scaffold for tissue engineering applications. Biomed Mater Eng 28:477–488. https://doi.org/10.3233/BME-171689
Mendoza-Novelo B, Cauich-Rodríguez JV (2009) The effect of surfactants, crosslinking agents and L-cysteine on the stabilization and mechanical properties of bovine pericardium. J Appl Biomater Biomech 7:123–131
Murphy CM, O'Brien FJ (2010) Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adh Migr 4:377–381. https://doi.org/10.4161/cam.4.3.11747
Neethling W, Brizard C, Firth L, Glancy R (2014) Biostability, durability and calcification of cryopreserved human pericardium after rapid glutaraldehyde-stabilization versus multistep ADAPT(R) treatment in a subcutaneous rat model. Eur J Cardiothorac Surg 45:e110–e117. https://doi.org/10.1093/ejcts/ezt623
Neethling WML, Puls K, Rea A (2018) Comparison of physical and biological properties of CardioCel® with commonly used bioscaffolds. Interact Cardiovasc Thorac Surg 26:985–992. https://doi.org/10.1093/icvts/ivx413
NIH (2011. Guide for the care and use of laboratory animals, 8th edn. National Academy of Sciences, Washington DC. https://www.ncbi.nlm.nih.gov/pubmed/21595115
Oswal D, Korossis S, Mirsadraee S, Wilcox H, Watterson K, Fisher J, Ingham E (2007) Biomechanical characterization of decellularized and cross-linked bovine pericardium. J Heart Valve Dis 16:165–174
Rémi E, Khelil N, Di Centa I, Roques C, Ba M, Medjahed-Hamidi F, Chaubet F, Letourneur D, Lansac E ,Meddahi-Pelle A (2011) Pericardial processing: challenges, outcomes and future prospects. In: Pignatello R (ed.) Biomaterials science and engineering. IntechOpen. https://www.intechopen.com/books/biomaterials-science-and-engineering/pericardial-processing-challenges-outcomes-and-future-prospects
Salameh A, Greimann W, Vondrys D, Kostelka M (2018) Calcification or not. This Is the Question. A 1-year study of bovine pericardial vascular patches (CardioCel) in minipigs. Semin Thorac Cardiovasc Surg 30:54–59. https://doi.org/10.1053/j.semtcvs.2017.09.013
Sasaki N, Odajima S (1996) Stress-strain curve and Young’s modulus of a collagen molecule as determined by the X-ray diffraction technique. J Biomech 29:655–658. https://doi.org/10.1016/0021-9290(95)00110-7
Schmidt CE, Baier JM (2000) Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials 21:2215–2231. https://doi.org/10.1016/s0142-9612(00)00148-4
Sommer G, Gasser TC, Regitnig P, Auer M, Holzapfel GA (2008) Dissection properties of the human aortic media: an experimental study. J Biomech Eng 130:021007. https://doi.org/10.1115/1.2898733
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43. https://doi.org/10.1016/s0022-5320(69)90033-1
Thubrikar MJ, Deck JD, Aouad J, Nolan SP (1983) Role of mechanical stress in calcification of aortic bioprosthetic valves. J Thorac Cardiovasc Surg 86:115–125
Umashankar PR, Mohanan PV, Kumari TV (2012) Glutaraldehyde treatment elicits toxic response compared to decellularization in bovine pericardium. Toxicol Int 19:51–58. https://doi.org/10.4103/0971-6580.94513
Wong ML, Wong JL, Vapniarsky N, Griffiths LG (2016) In vivo xenogeneic scaffold fate is determined by residual antigenicity and extracellular matrix preservation. Biomaterials 92:1–12. https://doi.org/10.1016/j.biomaterials.2016.03.024
Acknowledgements
We would like to thank the Department of Anatomical Pathology, UFS, specifically Prof J Goedhals and the Centre for Confocal and Electron Microscopy, UFS.
Funding
The study was funded by the Department Cardiothoracic Surgery, University of the Free State, Bloemfontein, South Africa.
Author information
Authors and Affiliations
Contributions
All authors contributed to the article and deserve authorship.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Ethics approval
The interfaculty Animal Ethics Committee of the University of the Free State (UFS-AED2015/0081) approved the study.
Consent for publication
We certify that all authors have agreed to be listed in the manuscript when submitted to peer review journals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Botes, L., Laker, L., Dohmen, P.M. et al. Advantages of decellularized bovine pericardial scaffolds compared to glutaraldehyde fixed bovine pericardial patches demonstrated in a 180-day implant ovine study. Cell Tissue Bank 23, 791–805 (2022). https://doi.org/10.1007/s10561-021-09988-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-021-09988-8