Abstract
Background
Recent large clinical trials have revealed that sodium–glucose cotransporter 2 (SGLT2) inhibitors improve cardiovascular outcomes not only in patients with heart failure with reduced ejection fraction, but also in patients with heart failure with mildly reduced or preserved ejection fraction (HFpEF). However, the effect of SGLT2 inhibitors on left ventricular (LV) diastolic function is still controversial.
Methods and Results
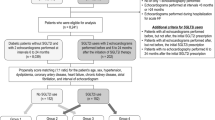
The TOP-HFPEF trial (Efficacy of Tofogliflozin on Left Ventricular Diastolic Dysfunction in Patients with Heart Failure with Preserved Ejection Fraction and Type 2 Diabetes Mellitus) is a multicenter, double-arm, open-label, confirmatory, investigator-initiated clinical study to investigate the effect of SGLT2 inhibitor on LV diastolic function in patients with HFpEF and type 2 diabetes mellitus. The participants are randomly assigned (1:1) to the tofogliflozin group (20 mg once daily) or the control group (administration or continuation of antidiabetic drugs other than SGLT2 inhibitors). The estimated number of patients to be enrolled in this trial is 90 in total (45 in each group). The participants are followed up for 52 weeks with tofogliflozin or control drugs. The primary endpoint is the change in E/e′ assessed by echocardiography from the baseline to the end of this study (52 weeks). This trial will also evaluate the effects of tofogliflozin on cardiovascular events, biomarkers, other echocardiographic parameters, the occurrence of atrial fibrillation, and renal function.
Conclusions
The TOP-HFPEF trial will clarify the efficacy of an SGLT2 inhibitor, tofogliflozin, on LV diastolic function in patients with HFpEF and type 2 diabetes mellitus.


Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Oren O, Goldberg S. Heart failure with preserved ejection fraction: diagnosis and management. Am J Med. 2017;130(5):510–6. https://doi.org/10.1016/j.amjmed.2016.12.031.
Nagai T, Yoshikawa T, Saito Y, et al. Clinical characteristics, management, and outcomes of Japanese patients hospitalized for heart failure with preserved ejection fraction - a report from the Japanese Heart Failure Syndrome with Preserved Ejection Fraction (JASPER) Registry. Circ J. 2018;82(6):1534–45. https://doi.org/10.1253/circj.CJ-18-0073.
Redfield MM, Jacobsen SJ, Burnett JC Jr, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194–202. https://doi.org/10.1001/jama.289.2.194.
Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–61. https://doi.org/10.1056/NEJMoa2107038.
Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387(12):1089–98. https://doi.org/10.1056/NEJMoa2206286.
McDonagh TA, Metra M, Adamo M, et al. (2023) Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023;44:3627–39. https://doi.org/10.1093/eurheartj/ehad195.
Otagaki M, Matsumura K, Kin H, et al. Effect of tofogliflozin on systolic and diastolic cardiac function in type 2 diabetic patients. Cardiovasc Drugs Ther. 2019;33(4):435–42. https://doi.org/10.1007/s10557-019-06892-y.
Lam CS, Roger VL, Rodeheffer RJ, et al. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County. Minnesota Circulation. 2007;115(15):1982–90. https://doi.org/10.1161/circulationaha.106.659763.
Dorfs S, Zeh W, Hochholzer W, et al. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J. 2014;35(44):3103–12. https://doi.org/10.1093/eurheartj/ehu315.
McDonagh TA, Metra M, Adamo M et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. European journal of heart failure. 2022;24(1):4–131. https://doi.org/10.1002/ejhf.2333
Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e876–94. https://doi.org/10.1161/cir.0000000000001062.
Okura H, Kubo T, Asawa K, et al. Elevated E/E’ predicts prognosis in congestive heart failure patients with preserved systolic function. Circ J. 2009;73(1):86–91. https://doi.org/10.1253/circj.cj-08-0457.
Komajda M, Isnard R, Cohen-Solal A, et al. Effect of ivabradine in patients with heart failure with preserved ejection fraction: the EDIFY randomized placebo-controlled trial. Eur J Heart Fail. 2017;19(11):1495–503. https://doi.org/10.1002/ejhf.876.
Carubelli V, Zhang Y, Metra M, et al. Treatment with 24 hour istaroxime infusion in patients hospitalised for acute heart failure: a randomised, placebo-controlled trial. Eur J Heart Fail. 2020;22(9):1684–93. https://doi.org/10.1002/ejhf.1743.
Suzuki Y, Kaneko H, Okada A, et al. Comparison of cardiovascular outcomes between SGLT2 inhibitors in diabetes mellitus. Cardiovasc Diabetol. 2022;21(1):67. https://doi.org/10.1186/s12933-022-01508-6.
Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383(15):1425–35. https://doi.org/10.1056/NEJMoa2004967.
Nikolaou PE, Mylonas N, Makridakis M, et al. Cardioprotection by selective SGLT-2 inhibitors in a non-diabetic mouse model of myocardial ischemia/reperfusion injury: a class or a drug effect? Basic Res Cardiol. 2022;117(1):27. https://doi.org/10.1007/s00395-022-00934-7.
Shim CY, Seo J, Cho I, et al. Randomized, controlled trial to evaluate the effect of dapagliflozin on left ventricular diastolic function in patients with type 2 diabetes mellitus: the IDDIA trial. Circulation. 2021;143(5):510–2. https://doi.org/10.1161/circulationaha.120.051992.
Prochaska JH, Jünger C, Schulz A, et al. Effects of empagliflozin on left ventricular diastolic function in addition to usual care in individuals with type 2 diabetes mellitus-results from the randomized, double-blind, placebo-controlled EmDia trial. Clinical research in cardiology : official journal of the German Cardiac Society. 2023;112(7):911–22. https://doi.org/10.1007/s00392-023-02164-w.
Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2016;29(4):277–314. https://doi.org/10.1016/j.echo.2016.01.011.
Borlaug BA, Reddy YNV, Braun A, et al. Cardiac and metabolic effects of dapagliflozin in heart failure with preserved ejection fraction: the CAMEO-DAPA Trial. Circulation. 2023;148(10):834–44. https://doi.org/10.1161/circulationaha.123.065134.
Akasaka H, Sugimoto K, Shintani A, et al. Effects of ipragliflozin on left ventricular diastolic function in patients with type 2 diabetes and heart failure with preserved ejection fraction: The EXCEED randomized controlled multicenter study. Geriatr Gerontol Int. 2022;22(4):298–304. https://doi.org/10.1111/ggi.14363.
Ejiri K, Miyoshi T, Kihara H, et al. Effect of luseogliflozin on heart failure with preserved ejection fraction in patients with diabetes mellitus. J Am Heart Assoc. 2020;9(16): e015103. https://doi.org/10.1161/jaha.119.015103.
Matsutani D, Sakamoto M, Kayama Y, et al. Effect of canagliflozin on left ventricular diastolic function in patients with type 2 diabetes. Cardiovasc Diabetol. 2018;17(1):73. https://doi.org/10.1186/s12933-018-0717-9.
Yu YW, Zhao XM, Wang YH, et al. Effect of sodium-glucose cotransporter 2 inhibitors on cardiac structure and function in type 2 diabetes mellitus patients with or without chronic heart failure: a meta-analysis. Cardiovasc Diabetol. 2021;20(1):25. https://doi.org/10.1186/s12933-020-01209-y.
Acknowledgements
We gratefully acknowledge Ms. Yumiko Ikeno and Ms. Ena Tsujita at Hanwa Memorial Hospital for their secretarial work, Ms. Kinuyo Ishida and Ms. Megumi Miyazaki for the construction of electrical case report form at National Cerebral and Cardiovascular Center.
Funding
This work was supported by Kowa Company, Limited.
Author information
Authors and Affiliations
Contributions
Study concept and design (SI, YN, HF, GN, MI, S Toyoda, S Takiuchi, TK, YI, YA, YS, ME, TI, MK); study conduction and patients’ recruitment (CI, GN, HY, HM, MI, S Toyoda, S Takiuchi, TK, YI, YA, MK); planned statistical analysis (SI, HF, TS, MK); writing (SI, YN, MK); figures and tables (SI, YN); advisor (YS, ME, TI). All authors read and approved this manuscript.
Corresponding author
Ethics declarations
Ethical Approval
This study is conducted in compliance with the Declaration of Helsinki and Japan’s Clinical Trials Act. The study protocol was approved by the Certified Review Board of Osaka Metropolitan University, and written informed consent will be obtained from all patients before their enrollment.
Consent to Participate
All eligible participants are required to provide written informed consent before enrollment in this study.
Consent for Publication
All authors reviewed the manuscript and approved its submission.
Conflict of Interest
Kowa Company will not have any role in the analysis or interpretation of the results of this study.
SI reports receiving a lecture fee from Nippon Boehringer Ingelheim Co., Ltd. and a grant from the Japan Society for the Promotion of Science, outside the submitted work.
YN reports receiving a grant from the Osaka Medical Research Foundation for Intractable Diseases, outside the submitted work.
HF reports receiving funds from Kitano Cadet-Doctor program, outside the submitted work.
CI reports personal fees from AstraZeneca K.K.; Medtronic Japan Co., Ltd.; Novartis Pharma K.K.; Kowa Co., Ltd.; Ono Pharmaceutical Co., Ltd.; Daiichi Sankyo Co., Ltd.; Bayer Yakuhin, Ltd.; Otsuka Pharmaceutical Co., Ltd.; Pfizer Japan Inc.; Sumitomo Pharma Co., Ltd.; Takeda Pharmaceutical Co., Ltd.; Edwards Lifesciences Corp.; Nippon Boehringer Ingelheim Co., Ltd.; Tsumura & Co.; Philips Japan, Ltd.; Eisai Co., Ltd.; Canon Medical Systems Corp.; Alnylam Japan K.K.; Janssen Pharmaceutical K.K.; Kyowa Kirin Co., Ltd.; Abbott Medical Japan LLC; Eli Lilly Japan K.K.; Astellas Pharma Inc.; Nihon Medi-Physics Co., Ltd.; and Japan Lifeline Co., Ltd., outside the submitted work.
GN has nothing to disclose.
HY has nothing to disclose.
HM has nothing to disclose.
MI reports receiving scholarship funds and personal fees from Daiichi Sankyo Co., Ltd.; Novartis Pharma K.K.; Nippon Shinyaku Co., Ltd.; Bayer Yakuhin, Ltd.; Mitsubishi Tanabe Pharma Corp.; Nippon Boehringer Ingelheim Co., Ltd.; and Otsuka Pharmaceutical Co., Ltd.; receiving scholarship funds from Teijin Pharma, Ltd.; Public Research Center; Nihon Medi-Physics Co., Ltd.; and Mochida Pharmaceutical Co., Ltd.; receiving joint research funds from Mie University, Research Institute for Production Development Japan, Janssen Pharmaceutical K.K., Nihon Kohden Corp., Nara Medical University, National Cerebral and Cardiovascular Center, and IQVIA Japan K.K.; receiving joint research funds and personal fees from Amgen Inc.; receiving personal fees from Kowa Co., Ltd.; Takeda Pharmaceutical Co., Ltd.; AstraZeneca K.K.; Pfizer Japan Inc.; Eli Lilly Japan K.K.; Ono Pharmaceutical Co., Ltd.; and Sumitomo Dainippon Pharma Co., Ltd., outside the submitted work.
S Toyoda reports receiving funds from Assist Japan Inc.; Abbott Vascular Japan Co., Ltd.; Abbott Medical Japan LLC; EP-CRSU Co., Ltd.; Next Japan Medicalnext Co., Ltd..; GE Healthcare Pharma Ltd.; CVQuest K.K.; Cmic Co., Ltd.; Sumitomo Electric Group CSR Foundation; Life Science Institute, Inc.; Teijin Pharma, Ltd.; Terumo Corp.; Nipro Corp.; Japan Heart Foundation; Next Japan Medicalnext Co., Ltd.; Japan Lifeline Co., Ltd.; Boston Scientific Japan K.K.; and Roche Diagnostics K.K.; receiving funds and lecture fees from Astellas Pharma Inc.; Otsuka Pharmaceutical Co., Ltd.; Ono Pharmaceutical Co., Ltd.; Kyowa Kirin Co., Ltd.; Kowa Co., Ltd.; Sanofi K.K.; Daiichi Sankyo Co., Ltd.; Sumitomo Pharma Co., Ltd.; Mitsubishi Tanabe Pharma Corp.; Tsumura & Co.; Abiomed Japan K.K.; Eli Lilly Japan K.K.; Nippon Shinyaku Co., Ltd.; Nippon Boehringer Ingelheim Co., Ltd.; Medtronic Japan Co., Ltd.; Novartis Pharma K.K.; and Mochida Pharmaceutical Co., Ltd.; and receiving lecture fees from Bayer Yakuhin, Ltd.; Viatris Inc.; Novo Nordisk Pharma, Ltd.; AstraZeneca K.K.; Takeda Pharmaceutical Co., Ltd.; Janssen Pharmaceutical K.K.; Fukuda Denshi Co., Ltd.; Protea Japan Co., Ltd.; Eisai Co., Ltd.; Edwards Lifesciences Corp.; Zeria Pharmaceutical Co., Ltd.; Pfizer Japan Inc.; Bristol Myers Squibb K.K.; MSD Inc.; Amicus Therapeutics Inc.; and Toa Eiyo, Ltd., outside the submitted work.
S Takiuchi has nothing to disclose.
TK reports receiving personal fees from Kaneka Corp. and Philips Japan, Ltd., and receiving lecture fees from Kowa Co., Ltd.; Otsuka Pharmaceutical Co., Ltd.; Novartis Pharma K.K.; Nippon Boehringer Ingelheim Co., Ltd.; Daiichi Sankyo Co., Ltd.; Ono Pharmaceutical Co., Ltd.; Tsumura & Co.; Toa Eiyo, Ltd.; AstraZeneca K.K.; Bayer Yakuhin, Ltd.; Boston Scientific Japan K.K.; Terumo Corp.; Nipro Corp.; and Abbott Medical Japan LLC, outside the submitted work.
YI reports receiving lecture fees from Pfizer Japan Inc.; Nippon Boehringer Ingelheim Co., Ltd.; Sanofi K.K.; Medtronic Japan Co., Ltd.; AstraZeneca K.K.; Toa Eiyo, Ltd.; Bayer Yakuhin, Ltd.; Takeda Pharmaceutical Co.; Ltd.; Sumitomo Pharma Co., Ltd.; Janssen Pharmaceutical K.K.; Otsuka Pharmaceutical Co., Ltd.; Kowa Co., Ltd.; Novartis Pharma K.K.; Mitsubishi Tanabe Pharma Corp.; Viatris Inc.; Nippon Shinyaku Co., Ltd.; Kyowa Kirin Co., Ltd.; Alnylam Japan K.K.; Eli Lilly Japan K.K.; JCR Pharmaceuticals Co., Ltd.; Amicus Therapeutics Inc.; Ono Pharmaceutical Co., Ltd.; Abbott Japan LLC; and GlaxoSmithKline Consumer Healthcare Japan K.K.; and receiving grants from Daiichi Sankyo Co., Ltd., outside the submitted work.
YA reports receiving personal fees from Abbott Medical Japan LLC; Astellas Pharma Inc.; AstraZeneca K.K.; Bayer Yakuhin, Ltd.; Daiichi Sankyo Co., Ltd.; Edwards Lifesciences Corp.; Eli Lilly Japan K.K.; GE Healthcare Japan Corp.; Mallinckrodt Pharma K.K.; Nippon Boehringer Ingelheim Co., Ltd.; Novartis Pharma K.K.; Ono Pharmaceutical Co., Ltd.; Otsuka Pharmaceutical Co., Ltd.; and Toa Eiyo, Ltd., outside the submitted work.
TS has nothing to disclose.
YS reports receiving personal fees from Daiichi Sankyo Co., Ltd.; Novartis Pharma K.K.; Nippon Boehringer Ingelheim Co., Ltd.; Otsuka Pharmaceutical Co., Ltd.; AstraZeneca K.K.; and Bayer Yakuhin, Ltd., outside the submitted work.
ME reports receiving grants from Mitsubishi Tanabe Pharma Corp.; Eisai Co., Ltd.; Chugai Pharmaceutical Co., Ltd.; and Bayer Yakuhin, Ltd.; receiving personal lecture fees from Novo Nordisk Pharma, Ltd.; Sanofi K.K.; AstraZeneca K.K.; MSD Inc.; Kowa Co., Ltd.; Teijin Pharma, Ltd.; Sanwa Co., Ltd.; Eli Lilly Japan K.K.; and Astellas Pharma Inc.; and receiving grants and personal lecture fees from Nippon Boehringer Ingelheim Co., Ltd.; Ono Pharmaceutical Co., Ltd.; Sumitomo Pharma Co., Ltd.; and Kyowa Kirin Co., Ltd., outside the submitted work.
TI reports personal fees from Kowa Co., Ltd.; Ono Pharmaceutical Co., Ltd.; Daiichi Sankyo Co., Ltd.; Mochida Pharmaceutical Co., Ltd.; Otsuka Pharmaceutical Co., Ltd.; AstraZeneca K.K.; Eisai Co., Ltd.; Mitsubishi Tanabe Pharma Corp.; Novo Nordic Pharma, Ltd.; and receiving grants from Fukuda Denshi Co., Ltd., outside the submitted work.
MK reports receiving personal fees from Daiichi Sankyo Co., Ltd.; Viatris Inc.; Otsuka Pharmaceutical Co., Ltd.; and Eli Lilly Japan K.K.; receiving grants and personal fees from Ono Pharmaceutical Co., Ltd.; Mitsubishi Tanabe Pharma Corp.; AstraZeneca K.K.; and Nippon Boehringer Ingelheim Co., Ltd.; receiving grants from Novartis Pharma K.K.; Takeda Pharmaceutical Co., Ltd.; and Kowa Co., Ltd., outside the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ito, S., Nakajima, Y., Fukuda, H. et al. Rationale and Design of Prospective, Multicenter, Double-Arm Clinical Trial to Investigate the Efficacy of Tofogliflozin on Left Ventricular Diastolic Dysfunction in Patients with Heart Failure with Preserved Ejection Fraction and Type 2 Diabetes Mellitus (TOP-HFPEF Trial). Cardiovasc Drugs Ther (2024). https://doi.org/10.1007/s10557-024-07576-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s10557-024-07576-y