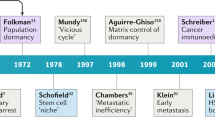
Abstract
The genomics and pathways governing metastatic dormancy are critically important drivers of long-term patient survival given the considerable portion of cancers that recur aggressively months to years after initial treatments. Our understanding of dormancy has expanded greatly in the last two decades, with studies elucidating that the dormant state is regulated by multiple genes, microenvironmental (ME) interactions, and immune components. These forces are exerted through mechanisms that are intrinsic to the tumor cell, manifested through cross-talk between tumor and ME cells including those from the immune system, and regulated by angiogenic processes in the nascent micrometastatic niche. The development of new in vivo and 3D ME models, as well as enhancements to decades-old tumor cell pedigree models that span the development of metastatic dormancy to aggressive growth, has helped fuel what arguably is one of the least understood areas of cancer biology that nonetheless contributes immensely to patient mortality. The current review focuses on the genes and molecular pathways that regulate dormancy via tumor-intrinsic and ME cells, and how groups have envisioned harnessing these therapeutically to benefit patient survival.

Similar content being viewed by others
References
Lambert, A. W., Pattabiraman, D. R., & Weinberg, R. A. (2017). Emerging biological principles of metastasis. Cell, 168(4), 670–691.
Aguirre-Ghiso, J. A. (2018). How dormant cancer persists and reawakens. Science, 361(6409), 1314–1315.
Banys-Paluchowski, M., Reinhardt, F., & Fehm, T. (2020). Disseminated tumor cells and dormancy in breast cancer progression. Advances in Experimental Medicine and Biology, 122035, 35–43.
Bushnell, G. G., Deshmukh, A. P., den Hollander, P., Luo, M., Soundararajan, R., Jia, D., Levine, H., Mani, S. A., & Wicha, M. S. (2021). Breast cancer dormancy: Need for clinically relevant models to address current gaps in knowledge. NPJ Breast Cancer, 7(1), 66.
Phan, T. G., & Croucher, P. I. (2020). The dormant cancer cell life cycle. Nature Reviews Cancer, 20(7), 398–411.
Gawrzak, S., Rinaldi, L., Gregorio, S., Arenas, E. J., Salvador, F., Urosevic, J., et al. (2018). MSK1 regulates luminal cell differentiation and metastatic dormancy in ER(+) breast cancer. Nature Cell Biology, 20(2), 211–221.
Keydar, I., Chen, L., Karby, S., Weiss, F. R., Delarea, J., Radu, M., Chaitcik, S., & Brenner, H. J. (1979). Establishment and characterization of a cell line of human breast carcinoma origin. European Journal of Cancer, 15(5), 659–670.
Harrell, J. C., Dye, W. W., Allred, D. C., Jedlicka, P., Spoelstra, N. S., Sartorius, C. A., & Horwitz, K. B. (2006). Estrogen receptor positive breast cancer metastasis: Altered hormonal sensitivity and tumor aggressiveness in lymphatic vessels and lymph nodes. Cancer Research, 66(18), 9308–9315.
Puchalapalli, M., Zeng, X., Mu, L., Anderson, A., Hix, G. L., Zhang, M., Sayyad, M. R., Mosticone, W. S., Clevenger, C. V., & Koblinski, J. E. (2016). NSG mice provide a better spontaneous model of breast cancer metastasis than athymic (Nude) mice. PLoS ONE, 11(9), e0163521.
Lefley, D., Howard, F., Arshad, F., Bradbury, S., Brown, H., Tulotta, C., Eyre, R., Alférez, D., Wilkinson, J. M., Holen, I., Clarke, R. B., & Ottewell, P. (2019). Development of clinically relevant in vivo metastasis models using human bone discs and breast cancer patient-derived xenografts. Breast Cancer Research, 21(1), 130–1220.
Lu, X., Mu, E., Wei, Y., Riethdorf, S., Yang, Q., Yuan, M., Yan, J., Hua, Y., Tiede, B. J., Lu, X., Haffty, B. G., Pantel, K., Massagué, J., & Kang, Y. (2011). VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell, 20(6), 701–714.
Albrengues, J., Shields, M. A., Ng, D., Park, C. G., Ambrico, A., Poindexter, M. E., et al. (2018). Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science, 361(6409), eaao4227.
Wada, M., Canals, D., Adada, M., Coant, N., Salama, M. F., Helke, K. L., Arthur, J. S., Shroyer, K. R., Kitatani, K., Obeid, L. M., & Hannun, Y. A. (2017). P38 delta MAPK promotes breast cancer progression and lung metastasis by enhancing cell proliferation and cell detachment. Oncogene., 36(47), 6649–6657.
Barkan, D., Kleinman, H., Simmons, J. L., Asmussen, H., Kamaraju, A. K., Hoenorhoff, M. J., Liu, Z. Y., Costes, S. V., Cho, E. H., Lockett, S., Khanna, C., Chambers, A. F., & Green, J. E. (2008). Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Research, 68(15), 6241–6250.
Rucci, N., Ricevuto, E., Ficorella, C., Longo, M., Perez, M., Di, G. C., Funari, A., Teti, A., & Migliaccio, S. (2004). In vivo bone metastases, osteoclastogenic ability, and phenotypic characterization of human breast cancer cells. Bone, 34(4), 697–709.
Sowder, M. E., & Johnson, R. W. (2018). Enrichment and detection of bone disseminated tumor cells in models of low tumor burden. Science Reports, 8(1), 14299.
Carlson, P., Dasgupta, A., Grzelak, C. A., Kim, J., Barrett, A., Coleman, I. M., Shor, R. E., Goddard, E. T., Dai, J., Schweitzer, E. M., Lim, A. R., Crist, S. B., Cheresh, D. A., Nelson, P. S., Hansen, K. C., & Ghajar, C. M. (2019). Targeting the perivascular niche sensitizes disseminated tumour cells to chemotherapy. Nature Cell Biology, 21(2), 238–250.
Holen, I., Walker, M., Nutter, F., Fowles, A., Evans, C. A., Eaton, C. L., & Ottewell, P. D. (2016). Oestrogen receptor positive breast cancer metastasis to bone: Inhibition by targeting the bone microenvironment in vivo. Clinical and Experimental Metastasis, 33(3), 211–224.
Malladi, S., Macalinao, D. G., Jin, X., He, L., Basnet, H., Zou, Y., De, S. E., & Massagué, J. (2016). Metastatic Latency and Immune Evasion through Autocrine Inhibition of WNT. Cell, 165(1), 45–60.
Ghajar, C. M., Peinado, H., Mori, H., Matei, I. R., Evason, K. J., Brazier, H., et al. (2013). The perivascular niche regulates breast tumour dormancy. Nature Cell Biology, 15(7), 807–817.
Gao, H., Chakraborty, G., Lee-Lim, A. P., Mo, Q., Decker, M., Vonica, A., Shen, R., Brogi, E., Brivanlou, A. H., & Giancotti, F. G. (2012). The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell, 150(4), 764–779.
Montagner, M., Bhome, R., Hooper, S., Chakravarty, P., Qin, X., Sufi, J., Bhargava, A., Ratcliffe, C. D. H., Naito, Y., Pocaterra, A., Tape, C. J., & Sahai, E. (2020). Crosstalk with lung epithelial cells regulates Sfrp2-mediated latency in breast cancer dissemination. Nature Cell Biology, 22(3), 289–296.
De Cock, J. M., Shibue, T., Dongre, A., Keckesova, Z., Reinhardt, F., & Weinberg, R. A. (2016). Inflammation triggers Zeb1-dependent escape from tumor latency. Cancer Research, 76(23), 6778–6784.
Piranlioglu, R., Lee, E., Ouzounova, M., Bollag, R. J., Vinyard, A. H., Arbab, A. S., Marasco, D., Guzel, M., Cowell, J. K., Thangaraju, M., Chadli, A., Hassan, K. A., Wicha, M. S., Celis, E., & Korkaya, H. (2019). Primary tumor-induced immunity eradicates disseminated tumor cells in syngeneic mouse model. Nature Communications, 10(1), 1430.
Luo, X. L., Deng, C. C., Su, X. D., Wang, F., Chen, Z., Wu, X. P., Liang, S. B., Liu, J. H., & Fu, L. W. (2018). Loss of MED12 induces tumor dormancy in human epithelial ovarian cancer via downregulation of EGFR. Cancer Research, 78(13), 3532–3543.
Liang, X., Gu, J., Li, T., Zhao, L., Fu, X., Zhang, W., Wang, J., Shang, Z., Huang, W., & Zhou, J. (2018). PAX5 haploinsufficiency induce cancer cell dormancy in Raji cells. Experimental Cell Research, 367(1), 30–36.
Kleinsmith, L. J., & Pierce, G. B., Jr. (1964). Multipotentiality of single embryonal carcinoma cells. Cancer Research, 24, 1544–1551.
Lawson, M. A., McDonald, M. M., Kovacic, N., Hua, K. W., Terry, R. L., Down, J., Kaplan, W., Paton-Hough, J., Fellows, C., Pettitt, J. A., Neil, D. T., Van, V. E., Baldock, P. A., Rogers, M. J., Eaton, C. L., Vanderkerken, K., Pettit, A. R., Quinn, J. M., Zannettino, A. C., … Croucher, P. I. (2015). Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche. Nature Communications, 6, 8983.
Chery, L., Lam, H. M., Coleman, I., Lakely, B., Coleman, R., Larson, S., Aguirre-Ghiso, J. A., Xia, J., Gulati, R., Nelson, P. S., Montgomery, B., Lange, P., Snyder, L. A., Vessella, R. L., & Morrissey, C. (2014). Characterization of single disseminated prostate cancer cells reveals tumor cell heterogeneity and identifies dormancy associated pathways. Oncotarget, 5(20), 9939–9951.
Sistigu, A., Musella, M., Galassi, C., Vitale, I., & De, M. R. (2020). Tuning cancer fate: Tumor microenvironment’s role in cancer stem cell quiescence and reawakening. Frontiers Immunology, 11, 2166.
Jahangiri, L., & Ishola, T. (2022). Dormancy in breast cancer, the role of autophagy, lncRNAs, miRNAs and exosomes. International Journal of Molecular Science, 23(9), 5271.
Korentzelos, D., Clark, A. M., & Wells, A. (2020). A perspective on therapeutic pan-resistance in metastatic cancer. International Journal of Molecular Science, 21(19), E7304.
Baram, T., Rubinstein-Achiasaf, L., Ben-Yaakov, H., & Ben-Baruch, A. (2021). Inflammation-driven breast tumor cell plasticity: Stemness/EMT, therapy resistance and dormancy. Frontiers Oncology, 10, 614468.
Smart, J. A., Oleksak, J. E., & Hartsough, E. J. (2021). Cell Adhesion Molecules in Plasticity and Metastasis. Molecular Cancer Research, 19(1), 25–37.
Dhaliwal, D., & Shepherd, T. G. (2022). Molecular and cellular mechanisms controlling integrin-mediated cell adhesion and tumor progression in ovarian cancer metastasis: A review. Clinical Experimental Metastasis, 39(2), 291–301.
Stuelten, C. H., Parent, C. A., & Montell, D. J. (2018). Cell motility in cancer invasion and metastasis: Insights from simple model organisms. Nature Reviews Cancer, 18(5), 296–312.
Genna, A., & Gil-Henn, H. (2018). FAK family kinases: The Yin and Yang of cancer cell invasiveness. Molecular and Cellular Oncology, 5(4), e1449584.
Zavyalova, M. V., Denisov, E. V., Tashireva, L. A., Savelieva, O. E., Kaigorodova, E. V., Krakhmal, N. V., & Perelmuter, V. M. (2019). Intravasation as a key step in cancer metastasis. Biochemistry (Mosc), 84(7), 762–772.
Tajbakhsh, A., Rivandi, M., Abedini, S., Pasdar, A., & Sahebkar, A. (2019). Regulators and mechanisms of anoikis in triple-negative breast cancer (TNBC): A review. Critical Reviews in Oncology and Hematology, 140, 17–27.
Adeshakin, F. O., Adeshakin, A. O., Afolabi, L. O., Yan, D., Zhang, G., & Wan, X. (2021). Mechanisms for modulating anoikis resistance in cancer and the relevance of metabolic reprogramming. Frontiers Oncology, 11, 626577.
Khan, S. U., Fatima, K., & Malik, F. (2022). Understanding the cell survival mechanism of anoikis-resistant cancer cells during different steps of metastasis. Clinical and Experimental Metastasis, 39(5), 715–726.
Liu, Y., Zhang, Y., Ding, Y., & Zhuang, R. (2021). Platelet-mediated tumor metastasis mechanism and the role of cell adhesion molecules. Critical Reviews in Oncology and Hematology, 167, 103502.
Reduzzi, C., Vismara, M., Gerratana, L., Silvestri, M., De, B. F., Raspagliesi, F., Verzoni, E., Di, C. S., Locati, L. D., Cristofanilli, M., Daidone, M. G., & Cappelletti, V. (2020). The curious phenomenon of dual-positive circulating cells: Longtime overlooked tumor cells. Seminars in Cancer Biology, 60, 344–350.
Hamilton, G., & Rath, B. (2017). Circulating tumor cell interactions with macrophages: Implications for biology and treatment. Translational Lung Cancer Research, 6(4), 418–430.
Banys, M., Krawczyk, N., & Fehm, T. (2014). The role and clinical relevance of disseminated tumor cells in breast cancer. Cancers (Basel), 6(1), 143–152.
Linde, N., Fluegen, G., & Aguirre-Ghiso, J. A. (2016). The relationship between dormant cancer cells and their microenvironment. Advances in Cancer Reseach, 13, 245–71.
Ring, A., Spataro, M., Wicki, A., & Aceto, N. (2022). Clinical and biological aspects of disseminated tumor cells and dormancy in breast cancer. Frontiers in Cell and Developmental Biology, 10, 929893.
Illyes, I., Tokes, A. M., Kovacs, A., Szasz, A. M., Molnar, B. A., Molnar, I. A., Kaszas, I., Baranyak, Z., Laszlo, Z., Kenessey, I., & Kulka, J. (2014). In breast cancer patients sentinel lymph node metastasis characteristics predict further axillary involvement. Virchows Archives, 465(1), 15–24.
Walter, S. D., Chao, D. L., Feuer, W., Schiffman, J., Char, D. H., & Harbour, J. W. (2016). Prognostic implications of tumor diameter in association with gene expression profile for uveal melanoma. JAMA Ophthalmology, 134(7), 734–740.
Wangchinda, P., & Ithimakin, S. (2016). Factors that predict recurrence later than 5 years after initial treatment in operable breast cancer. World Journal of Surgical Oncology, 14(1), 223–0988.
Sopik, V., & Narod, S. A. (2018). The relationship between tumour size, nodal status and distant metastases: On the origins of breast cancer. Breast Cancer Research and Treatments, 170(3), 647–656.
Asare, E. A., Silva-Figueroa, A., Hess, K. R., Busaidy, N., Graham, P. H., Grubbs, E. G., Lee, J. E., Williams, M. D., & Perrier, N. D. (2019). Risk of distant metastasis in parathyroid carcinoma and its effect on survival: A retrospective review from a high-volume center. Annals of Surgical Oncology, 26(11), 3593–3599.
Holmberg, L., Bill-Axelson, A., Helgesen, F., Salo, J. O., Folmerz, P., Häggman, M., Andersson, S. O., Spångberg, A., Busch, C., Nordling, S., Palmgren, J., Adami, H. O., Johansson, J. E., & Norlén, B. J. (2002). A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. New England Journal of Medicine, 347(11), 781–789.
Fidler, I. J., & Kripke, M. L. (1977). Metastasis results from preexisting variant cells within a malignant tumor. Science, 197(4306), 893–895.
Kang, Y., Siegel, P. M., Shu, W., Drobnjak, M., Kakonen, S. M., Cordon-Cardo, C., Guise, T. A., & Massagué, J. (2003). A multigenic program mediating breast cancer metastasis to bone. Cancer Cell, 3(6), 537–549.
Va’nt Veer, L. J., Dai, H., van de Vijver, M. J., He, Y. D., Hart, A. A., Mao, M., Peterse, H. L., van der Kooy, K., Marton, M. J., Witteveen, A. T., Schreiber, G. J., Kerkhoven, R. M., Roberts, C., Linsley, P. S., Bernards, R., & Friend, S. H. (2002). Gene expression profiling predicts clinical outcome of breast cancer. Nature, 415(6871), 530–536.
Patsialou, A., Wang, Y., Lin, J., Whitney, K., Goswami, S., Kenny, P. A., & Condeelis, J. S. (2012). Selective gene-expression profiling of migratory tumor cells in vivo predicts clinical outcome in breast cancer patients. Breast Cancer Research, 14(5), R139.
Kato, S., Alsafar, A., Walavalkar, V., Hainsworth, J., & Kurzrock, R. (2021). Cancer of unknown primary in the molecular era. Trends in Cancer, 7(5), 465–477.
Schmidt-Kittler, O., Ragg, T., Daskalakis, A., Granzow, M., Ahr, A., Blankenstein, T. J., Kaufmann, M., Diebold, J., Arnholdt, H., Muller, P., Bischoff, J., Harich, D., Schlimok, G., Riethmuller, G., Eils, R., & Klein, C. A. (2003). From latent disseminated cells to overt metastasis: Genetic analysis of systemic breast cancer progression. Proceedings of the National Academy of Sciences, USA, 100(13), 7737–7742.
Hüsemann, Y., Geigl, J. B., Schubert, F., Musiani, P., Meyer, M., Burghart, E., Forni, G., Eils, R., Fehm, T., Riethmüller, G., & Klein, C. A. (2008). Systemic spread is an early step in breast cancer. Cancer Cell, 13(1), 58–68.
Schardt, J. A., Meyer, M., Hartmann, C. H., Schubert, F., Schmidt-Kittler, O., Fuhrmann, C., Polzer, B., Petronio, M., Eils, R., & Klein, C. A. (2005). Genomic analysis of single cytokeratin-positive cells from bone marrow reveals early mutational events in breast cancer. Cancer Cell, 8(3), 227–239.
Harper, K. L., Sosa, M. S., Entenberg, D., Hosseini, H., Cheung, J. F., Nobre, R., Avivar-Valderas, A., Nagi, C., Girnius, N., Davis, R. J., Farias, E. F., Condeelis, J., Klein, C. A., & Aguirre-Ghiso, J. A. (2016). Mechanism of early dissemination and metastasis in Her2+ mammary cancer. Nature, 540588-592.
Fox, D. B., Garcia, N. M. G., McKinney, B. J., Lupo, R., Noteware, L. C., Newcomb, R., Liu, J., Locasale, J. W., Hirschey, M. D., & Alvarez, J. V. (2020). NRF2 activation promotes the recurrence of dormant tumour cells through regulation of redox and nucleotide metabolism. Nature Metabolism, 2(4), 318–334.
Hartkopf, A. D., Brucker, S. Y., Taran, F. A., Harbeck, N., von Au, A., Naume, B., et al. (2021). Disseminated tumour cells from the bone marrow of early breast cancer patients: Results from an international pooled analysis. European Journal of Cancer, 154, 128–137.
Popawski, A. B., Jankowski, M., Erickson, S. W., de Díaz, S. T., Partridge, E. C., Crasto, C., et al. (2010). Frequent genetic differences between matched primary and metastatic breast cancer provide an approach to identification of biomarkers for disease progression. European Journal of Human Genetics, 18(5), 560–568.
Stoecklein, N. H., & Klein, C. A. (2010). Genetic disparity between primary tumours, disseminated tumour cells, and manifest metastasis. International Journal of Cancer, 126(3), 589–598.
Gundem, G., Van, L. P., Kremeyer, B., Alexandrov, L. B., Tubio, J. M., Papaemmanuil, E., et al. (2015). The evolutionary history of lethal metastatic prostate cancer. Nature, 520(7547), 353–357.
Cackowski, F. C., Wang, Y., Decker, J. T., Sifuentes, C., Weindorf, S., Jung, Y., et al. (2019). Detection and isolation of disseminated tumor cells in bone marrow of patients with clinically localized prostate cancer. Prostate, 79(14), 1715–1727.
Werner-Klein, M., Scheitler, S., Hoffmann, M., Hodak, I., Dietz, K., Lehnert, P., et al. (2018). Genetic alterations driving metastatic colony formation are acquired outside of the primary tumour in melanoma. Nature Communications, 9(1), 595.
Werner-Klein, M., Grujovic, A., Irlbeck, C., Obradovic, M., Hoffmann, M., Koerkel-Qu, H., et al. (2020). Interleukin-6 trans-signaling is a candidate mechanism to drive progression of human DCCs during clinical latency. Nature Communications, 11(1), 4977–18701.
Tohme, S., Simmons, R. L., & Tsung, A. (2017). Surgery for cancer: A trigger for metastases. Cancer Research, 77(7), 1548–1552.
Aguirre-Ghiso, J. A., Estrada, Y., Liu, D., & Ossowski, L. (2003). ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK). Cancer Research, 63(7), 1684–1695.
Yanagisawa, M., Huveldt, D., Kreinest, P., Lohse, C. M., Cheville, J. C., Parker, A. S., Copland, J. A., & Anastasiadis, P. Z. (2008). A p120 catenin isoform switch affects Rho activity, induces tumor cell invasion, and predicts metastatic disease. Journal of Biological Chemistry, 283(26), 18344–18354.
Yumoto, K., Eber, M. R., Berry, J. E., Taichman, R. S., & Shiozawa, Y. (2014). Molecular pathways: Niches in metastatic dormancy. Clinical Cancer Research, 20(13), 3384–3389.
Adam, A. P., George, A., Schewe, D., Bragado, P., Iglesias, B. V., Ranganathan, A. C., Kourtidis, A., Conklin, D. S., & Aguirre-Ghiso, J. A. (2009). Computational identification of a p38SAPK-regulated transcription factor network required for tumor cell quiescence. Cancer Research, 69(14), 5664–5672.
Yang, X., Wu, J. S., Li, M., Zhang, W. L., Gao, X. L., Wang, H. F., et al. (2021). Inhibition of DEC2 is necessary for exiting cell dormancy in salivary adenoid cystic carcinoma. Journal of Experimental Clinical Cancer Research, 40(1), 169–01956.
McGrath, J. E., Panzica, L., Ransom, R., Withers, H. G., & Gelman, I. H. (2019). Identification of genes regulating breast cancer dormancy in 3D bone endosteal niche cultures. Molecular Cancer Research, 17(4), 1541–7786.
Guereño, M., Delgado, P. M., Lugones, A. C., Cercato, M., Todaro, L., Urtreger, A., & Peters, M. G. (2020). Glypican-3 (GPC3) inhibits metastasis development promoting dormancy in breast cancer cells by p38 MAPK pathway activation. European Journal of Cell Biology, 99(6), 151096.
Schewe, D. M., & Aguirre-Ghiso, J. A. (2008). ATF6alpha-Rheb-mTOR signaling promotes survival of dormant tumor cells in vivo. Proceedings of the National Academy of Sciences, USA, 105(30), 10519–10524.
Yamaguchi, M. (2005). Role of regucalcin in maintaining cell homeostasis and function (review). International Journal of Molecular Medicine, 15(3), 371–389.
Sharma, S., Pei, X., Xing, F., Wu, S. Y., Wu, K., Tyagi, A., Zhao, D., Deshpande, R., Ruiz, M. G., Singh, R., Lyu, F., & Watabe, K. (2020). Regucalcin promotes dormancy of prostate cancer. Oncogene, 40(5), 1012–1026.
Horak, C. E., Lee, J. H., Marshall, J. C., Shreeve, S. M., & Steeg, P. S. (2008). The role of metastasis suppressor genes in metastatic dormancy. APMIS., 116(7–8), 586–601.
Gelman, I. H. (2012). Suppression of tumor and metastasis progression through the scaffolding functions of SSeCKS/Gravin/AKAP12. Cancer Metastasis Review, 31(3–4), 493–500.
Kim, R. S., Avivar-Valderas, A., Estrada, Y., Bragado, P., Sosa, M. S., Aguirre-Ghiso, J. A., & Segall, J. E. (2012). Dormancy signatures and metastasis in estrogen receptor positive and negative breast cancer. PLoS ONE, 7(4), e35569.
Lawson, D. A., Bhakta, N. R., Kessenbrock, K., Prummel, K. D., Yu, Y., Takai, K., Zhou, A., Eyob, H., Balakrishnan, S., Wang, C. Y., Yaswen, P., Goga, A., & Werb, Z. (2015). Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature, 526(7571), 131–135.
Gao, H., Chakraborty, G., Lee-Lim, A. P., Mavrakis, K. J., Wendel, H. G., & Giancotti, F. G. (2014). Forward genetic screens in mice uncover mediators and suppressors of metastatic reactivation. Proceedings of the National Academy of Sciences, U.S.A, 111(46), 16532–16537.
Dudgeon, C., Harris, C. R., Chen, Y., Ghaddar, B., Sharma, A., Shah, M. M., Roberts, A. I., Casabianca, A., Collisson, E. A., Balachandran, V. P., Vertino, P. M., De, S., & Carpizo, D. R. (2020). A novel model of pancreatic cancer dormancy reveals mechanistic insights and a dormancy gene signature with human relevance. BioRxiv. https://doi.org/10.1101/2020.04.13.037374
Uzuner, D., Akkoç, Y., Peker, N., Pir, P., Gözüaçik, D., & Çakir, T. (2021). Transcriptional landscape of cellular networks reveal interactions driving the dormancy mechanisms in cancer. Science Reports, 11(1), 15806–94005.
Quayle, L. A., Spicer, A., Ottewell, P. D., & Holen, I. (2021). Transcriptomic profiling reveals novel candidate genes and signalling programs in breast cancer quiescence and dormancy. Cancers (Basel), 13(16), 3922.
Ren, Q., Khoo, W. H., Corr, A. P., Phan, T. G., Croucher, P. I., & Stewart, S. A. (2022). Gene expression predicts dormant metastatic breast cancer cell phenotype. Breast Cancer Research, 24(1), 10.
Janghorban, M., Yang, Y., Zhao, N., Hamor, C., Nguyen, T. M., Zhang, X. H., & Rosen, J. M. (2022). Single-Cell analysis unveils the role of the tumor immune microenvironment and notch signaling in dormant minimal residual disease. Cancer Research, 82(5), 885–899.
Son, J., Lee, J. H., Kim, H. N., Ha, H., & Lee, Z. H. (2010). cAMP-response-element-binding protein positively regulates breast cancer metastasis and subsequent bone destruction. Biochemical and Biophysical Research Communications, 398(2), 309–314.
Johnson, R. W., Sun, Y., Ho, P. W. M., Chan, A. S. M., Johnson, J. A., Pavlos, N. J., Sims, N. A., & Martin, T. J. (2018). Parathyroid hormone-related protein negatively regulates tumor cell dormancy genes in a PTHR1/cyclic AMP-independent manner. Frontiers in Endocrinology (Lausanne), 9, 241.
La Belle, F. A., Calhoun, B. C., Sharma, A., Chang, J. C., Almasan, A., & Schiemann, W. P. (2019). Autophagy inhibition elicits emergence from metastatic dormancy by inducing and stabilizing Pfkfb3 expression. Nature Communications, 10(1), 3668–11640.
Blessing, A. M., Santiago-O’Farrill, J. M., Mao, W., Pang, L., Ning, J., Pak, D., et al. (2020). Elimination of dormant, autophagic ovarian cancer cells and xenografts through enhanced sensitivity to anaplastic lymphoma kinase inhibition. Cancer, 126(15), 3579–3592.
Onoyama, I., & Nakayama, K. I. (2008). Fbxw7 in cell cycle exit and stem cell maintenance: Insight from gene-targeted mice. Cell Cycle, 7(21), 3307–3313.
Iriuchishima, H., Takubo, K., Matsuoka, S., Onoyama, I., Nakayama, K. I., Nojima, Y., & Suda, T. (2011). Ex vivo maintenance of hematopoietic stem cells by quiescence induction through Fbxw7a; overexpression. Blood, 117(8), 2373–2377.
Jiang, J., Zheng, M., Zhang, M., Yang, X., Li, L., Wang, S. S., Wu, J. S., Yu, X. H., Wu, J. B., Pang, X., Tang, Y. J., Tang, Y. L., & Liang, X. H. (2019). PRRX1 regulates cellular phenotype plasticity and dormancy of head and neck squamous cell carcinoma through miR-642b-3p. Neoplasia., 21(2), 216–229.
Sosa, M. S., Parikh, F., Maia, A. G., Estrada, Y., Bosch, A., Bragado, P., et al. (2015). NR2F1 controls tumour cell dormancy via SOX9- and RARbeta-driven quiescence programmes. Nature Communications, 6, 6170.
Satcher, R. L., & Zhang, X. H. (2021). Evolving cancer-niche interactions and therapeutic targets during bone metastasis. Nat Rev Cancer, 22(2), 85–101.
Fluegen, G., Avivar-Valderas, A., Wang, Y., Padgen, M. R., Williams, J. K., Nobre, A. R., et al. (2017). Phenotypic heterogeneity of disseminated tumour cells is preset by primary tumour hypoxic microenvironments. Nature Cell Biology, 19(2), 120–132.
Ju, S., Wang, F., Wang, Y., & Ju, S. (2020). CSN8 is a key regulator in hypoxia-induced epithelial-mesenchymal transition and dormancy of colorectal cancer cells. Molecular Cancer, 19(1), 168–01285.
Wu, R., Roy, A. M., Tokumaru, Y., Gandhi, S., Asaoka, M., Oshi, M., Yan, L., Ishikawa, T., & Takabe, K. (2022). NR2F1, a tumor dormancy marker, is expressed predominantly in cancer-associated fibroblasts and is associated with suppressed breast cancer cell proliferation. Cancers (Basel), 14(12), 2962.
Sanchez, C. A., Yamamoto, T., Kawamura, Y., Hironaka-Mitsuhashi, A., Ono, M., Tsuda, H., Shimomura, A., Tamura, K., Takeshita, F., Ochiya, T., & Yamamoto, Y. (2020). Long non-coding NR2F1-AS1 is associated with tumor recurrence in estrogen receptor-positive breast cancers. Molecular Oncology, 14(9), 2271–2287.
Liu, Y., Zhang, P., Wu, Q., Fang, H., Wang, Y., Xiao, Y., Cong, M., Wang, T., He, Y., Ma, C., Tian, P., Liang, Y., Qin, L. X., Yang, Q., Yang, Q., Liao, L., & Hu, G. (2021). Long non-coding RNA NR2F1-AS1 induces breast cancer lung metastatic dormancy by regulating NR2F1 and DNp63. Nature Communications, 12(1), 5232.
Zhu, X., Wang, F., Wu, X., Li, Z., Wang, Z., Ren, X., Zhou, Y., Song, F., Liang, Y., Zeng, Z., Liao, W., Ding, Y., Liao, W., & Liang, L. (2020). FBX8 promotes metastatic dormancy of colorectal cancer in liver. Cell Death and Disease, 11(8), 622–02870.
Gooding, A. J., & Schiemann, W. P. (2020). Epithelial-mesenchymal transition programs and cancer stem cell phenotypes: Mediators of breast cancer therapy resistance. Molecular Cancer Research, 18(9), 1257–1270.
Johnson, R. W., Finger, E. C., Olcina, M. M., Vilalta, M., Aguilera, T., Miao, Y., et al. (2016). Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow. Nature Cell Biology, 18(10), 1078–1089.
Clements, M. E., Holtslander, L., Edwards, C., Todd, V., Dooyema, S. D. R., Bullock, K., Bergdorf, K., Zahnow, C. A., Connolly, R. M., & Johnson, R. W. (2021). HDAC inhibitors induce LIFR expression and promote a dormancy phenotype in breast cancer. Oncogene, 40(34), 5314–5324.
Bragado, P., Estrada, Y., Parikh, F., Krause, S., Capobianco, C., Farina, H. G., Schewe, D. M., & Aguirre-Ghiso, J. A. (2013). TGF-beta2 dictates disseminated tumour cell fate in target organs through TGF-beta-RIII and p38alpha/beta signalling. Nature Cell Biology, 15(11), 1351–1361.
Ribatti, D., Mangialardi, G., & Vacca, A. (2006). Stephen Paget and the ‘seed and soil’ theory of metastatic dissemination. Clinical Experimental Medicine, 6(4), 145–149.
Ruppender, N., Larson, S., Lakely, B., Kollath, L., Brown, L., Coleman, I., et al. (2015). Cellular adhesion promotes prostate cancer cells escape from dormancy. PLoS ONE, 10(6), e0130565.
Yumoto, K., Eber, M. R., Wang, J., Cackowski, F. C., Decker, A. M., Lee, E., Nobre, A. R., Aguirre-Ghiso, J. A., Jung, Y., & Taichman, R. S. (2016). Axl is required for TGF-beta2-induced dormancy of prostate cancer cells in the bone marrow. Sci Reports, 6, 36520.
Burstyn-Cohen, T., & Maimon, A. (2019). TAM receptors, phosphatidylserine, inflammation, and cancer. Cell Communication and Signaling, 17(1), 156–0461.
Axelrod, H. D., Valkenburg, K. C., Amend, S. R., Hicks, J. L., Parsana, P., Torga, G., DeMarzo, A. M., & Pienta, K. J. (2018). AXL is a putative tumor suppressor and dormancy regulator in prostate cancer. Molecular Cancer Research, 17(2), 356–369.
Cackowski, F. C., Eber, M. R., Rhee, J., Decker, A., Yumoto, K., Berry, J. E., Lee, E., Shiozawa, Y., Jung, Y., Aguirre-Ghiso, J. A., & Taichman, R. S. (2016). Mer tyrosine kinase regulates disseminated prostate cancer cellular dormancy. Journal of Cell Biochemistry, 118(4), 891–902.
Kobayashi, A., Okuda, H., Xing, F., Pandey, P. R., Watabe, M., Hirota, S., Pai, S. K., Liu, W., Fukuda, K., Chambers, C., Wilber, A., & Watabe, K. (2011). Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. Journal of Experimental Medicine, 208(13), 2641–2655.
Eckhardt, B. L., Cao, Y., Redfern, A. D., Chi, L. H., Burrows, A. D., Roslan, S., Sloan, E. K., Parker, B. S., Loi, S., Ueno, N. T., Lau, P. K. H., Latham, B., & Anderson, R. L. (2020). Activation of canonical BMP4-SMAD7 signaling suppresses breast cancer metastasis. Cancer Research, 80(6), 1304–1315.
Nobre, A. R., Risson, E., Singh, D. K., Di Martino, J. S., Cheung, J. F., Wang, J., Johnson, J., Russnes, H. G., Bravo-Cordero, J. J., Birbrair, A., Naume, B., Azhar, M., Frenette, P. S., & Aguirre-Ghiso, J. A. (2021). Bone marrow NG2(+)/Nestin(+) mesenchymal stem cells drive DTC dormancy via TGFb2. Nature Cancer, 2(3), 327–339.
Prunier, C., Baker, D., Ten, D. P., & Ritsma, L. (2019). TGF-b family signaling pathways in cellular dormancy. Trends in Cancer, 5(1), 66–78.
Sharma, S., Xing, F., Liu, Y., Wu, K., Said, N., Pochampally, R., Shiozawa, Y., Lin, H. K., Balaji, K. C., & Watabe, K. (2016). Secreted protein acidic and rich in cysteine (SPARC) mediates metastatic dormancy of prostate cancer in bone. Journal of Biological Chemistry, 291(37), 19351–19363.
Cassar, L., Li, H., Pinto, A. R., Nicholls, C., Bayne, S., & Liu, J. P. (2008). Bone morphogenetic protein-7 inhibits telomerase activity, telomere maintenance, and cervical tumor growth. Cancer Research, 68(22), 9157–9166.
Nahm, J. H., Yang, W. I., & Yoon, S. O. (2020). Forkhead Box C1 (FOXC1) Expression in stromal cells within the microenvironment of T and NK Cell Lymphomas: Association with tumor dormancy and activation. Cancer Research and Treatments, 52(4), 1273–1282.
Zangrossi, M., Romani, P., Chakravarty, P., Ratcliffe, C. D. H., Hooper, S., Dori, M., Forcato, M., Bicciato, S., Dupont, S., Sahai, E., & Montagner, M. (2021). EphB6 regulates TFEB-lysosomal pathway and survival of disseminated indolent breast cancer cells. Cancers (Basel), 13(5), 1079.
Visan, K. S., Lobb, R. J., & Moller, A. (2020). The role of exosomes in the promotion of epithelial-to-mesenchymal transition and metastasis. Frontiers in Bioscience (Landmark Ed.), 251022-1057.
Attaran, S., & Bissell, M. J. (2021). The role of tumor microenvironment and exosomes in dormancy and relapse. Seminars in Cancer Biology, 78, 35–44.
Ono, M., Kosaka, N., Tominaga, N., Yoshioka, Y., Takeshita, F., Takahashi, R. U., Yoshida, M., Tsuda, H., Tamura, K., & Ochiya, T. (2014). Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Science Signaling, 7(332), ra63.
Li, X., Yang, J., Bao, M., Zeng, K., Fu, S., Wang, C., & Ye, L. (2018). Wnt signaling in bone metastasis: Mechanisms and therapeutic opportunities. Life Sciences, 208, 33–45.
Ren, D., Dai, Y., Yang, Q., Zhang, X., Guo, W., Ye, L., Huang, S., Chen, X., Lai, Y., Du, H., Lin, C., Peng, X., & Song, L. (2019). Wnt5a induces and maintains prostate cancer cells dormancy in bone. Journal of Experimental Medicine, 216(2), 428–449.
Fane, M. E., Chhabra, Y., Alicea, G. M., Maranto, D. A., Douglass, S. M., Webster, M. R., et al. (2022). Stromal changes in the aged lung induce an emergence from melanoma dormancy. Nature, 606(7913), 396–405.
Lee, E., Yang, J., Ku, M., Kim, N. H., Park, Y., Park, C. B., Suh, J. S., Park, E. S., Yook, J. I., Mills, G. B., Huh, Y. M., & Cheong, J. H. (2015). Metabolic stress induces a Wnt-dependent cancer stem cell-like state transition. Cell Death and Disease, 6, e1805.
Bragado, P., Estrada, Y., Parikh, F., Krause, S., Capobianco, C., Farina, H. G., Schewe, D. M., & Aguirre-Ghiso, J. A. (2013). TGF-b dictates disseminated tumour cell fate in target organs through TGF-b-RIII and p38a signalling. Nature Cell Biology, 15(11), 1351–1361.
Hernandez, S., Serrano, A. G., & Solis Soto, L. M. (2022). The role of nerve fibers in the tumor immune microenvironment of solid tumors. Advanced Biology (Weinh), 6(9), e2200046.
Erin, N., Shurin, G. V., Baraldi, J. H., & Shurin, M. R. (2022). Regulation of carcinogenesis by sensory neurons and neuromediators. Cancers (Basel), 14(9), 2333.
Roda, N., Blandano, G., & Pelicci, P. G. (2021). Blood vessels and peripheral nerves as key players in cancer progression and therapy resistance. Cancers (Basel), 13(17), 4471.
Mulcrone, P. L., Campbell, J. P., Clément-Demange, L., Anbinder, A. L., Merkel, A. R., Brekken, R. A., Sterling, J. A., & Elefteriou, F. (2017). Skeletal colonization by breast cancer cells is stimulated by an osteoblast and b2AR-dependent neo-angiogenic switch. Journal of Bone Mineral Research, 32(7), 1442–1454.
Dai, J., Cimino, P. J., Gouin, K. H., III., Grzelak, C. A., Barrett, A., Lim, A. R., et al. (2022). Astrocytic laminin-211 drives disseminated breast tumor cell dormancy in brain. Nature Cancer, 3(1), 25–42.
Ni, B., Li, Q., Zhuang, C., Huang, P., Xia, X., Yang, L., Ma, X., Huang, C., Zhao, W., Tu, L., Shen, Y., Zhu, C., Zhang, Z., Zhao, E., Wang, M., & Cao, H. (2022). The nerve-tumour regulatory axis GDNF-GFRA1 promotes tumour dormancy, imatinib resistance and local recurrence of gastrointestinal stromal tumours by achieving autophagic flux. Cancer Letters, 535, 215639.
Touil, Y., Segard, P., Ostyn, P., Begard, S., Aspord, C., El Machhour, M. R., et al. (2016). Melanoma dormancy in a mouse model is linked to GILZ/FOXO3A-dependent quiescence of disseminated stem-like cells. Science Reports, 6, 30405.
Liburkin-Dan, T., Toledano, S., & Neufeld, G. (2022). Lysyl oxidase family enzymes and their role in tumor progression. Internatinal Journal of Molecular Science, 23(11), 6249.
Ferreira, S., Saraiva, N., Rijo, P., & Fernandes, A. S. (2021). LOXL2 inhibitors and breast cancer progression. Antioxidants (Basel), 10(2), 312.
Weidenfeld, K., Schif-Zuck, S., Abu-Tayeh, H., Kang, K., Kessler, O., Weissmann, M., Neufeld, G., & Barkan, D. (2016). Dormant tumor cells expressing LOXL2 acquire a stem-like phenotype mediating their transition to proliferative growth. Oncotarget, 7(44), 71362–71377.
Aslakson, C. J., & Miller, F. R. (1992). Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Research, 52(6), 1399–1405.
Almog, N., Ma, L., Raychowdhury, R., Schwager, C., Erber, R., Short, S., Hlatky, L., Vajkoczy, P., Huber, P. E., Folkman, J., & Abdollahi, A. (2009). Transcriptional switch of dormant tumors to fast-growing angiogenic phenotype. Cancer Research, 69(3), 836–844.
Tiram, G., Segal, E., Krivitsky, A., Shreberk-Hassidim, R., Ferber, S., Ofek, P., et al. (2016). Identification of dormancy-associated MicroRNAs for the design of osteosarcoma-targeted dendritic polyglycerol nanopolyplexes. ACS Nanotechnology, 10(2), 2028–2045.
Owen, K. L., Gearing, L. J., Zanker, D. J., Brockwell, N. K., Khoo, W. H., Roden, D. L., et al. (2020). Prostate cancer cell-intrinsic interferon signaling regulates dormancy and metastatic outgrowth in bone. EMBO Reports, 21(6), e50162.
Power, C. A., Pwint, H., Chan, J., Cho, J., Yu, Y., Walsh, W., & Russell, P. J. (2009). A novel model of bone-metastatic prostate cancer in immunocompetent mice. Prostate, 69(15), 1613–1623.
Wang, X., Yu, J., Yan, J., Peng, K., & Zhou, H. (2022). Single-cell sequencing reveals MYC targeting gene MAD2L1 is associated with prostate cancer bone metastasis tumor dormancy. BMC Urology, 22(1), 37.
Kudaravalli, S., den Hollander, P., & Mani, S. A. (2022). Role of p38 MAP kinase in cancer stem cells and metastasis. Oncogene, 41(23), 3177–3185.
Qin, J. Z., Xin, H., Qi, X. M., & Chen, G. (2022). Isoform-specific and cell/tissue-dependent effects of p38 MAPKs in regulating inflammation and inflammation-associated oncogenesis. Frontiers in Bioscience (Landmark Ed.), 27(1), 31.
Ohkubo, S., Nakahata, N., & Ohizumi, Y. (1996). Thromboxane A2-mediated shape change: Independent of Gq-phospholipase C-Ca2+ pathway in rabbit platelets. British Journal of Pharmacology, 117(6), 1095–1104.
Janowska, A., Iannone, M., Fidanzi, C., Romanelli, M., Filippi, L., Del, R. M., Martins, M., & Dini, V. (2022). The genetic basis of dormancy and awakening in cutaneous metastatic melanoma. Cancers (Basel), 14(9), 2104.
Asghar, U., Witkiewicz, A. K., Turner, N. C., & Knudsen, E. S. (2015). The history and future of targeting cyclin-dependent kinases in cancer therapy. Nature Reviews Drug Discovery, 14(2), 130–146.
Singh, D. K., Patel, V. G., Oh, W. K., & Aguirre-Ghiso, J. A. (2021). Prostate cancer dormancy and reactivation in bone marrow. Journal of Clinical Medicine, 10(12), 2648.
Ramamoorthi, G., Kodumudi, K., Gallen, C., Zachariah, N. N., Basu, A., Albert, G., Beyer, A., Snyder, C., Wiener, D., Costa, R. L. B., & Czerniecki, B. J. (2021). Disseminated cancer cells in breast cancer: mechanism of dissemination and dormancy and emerging insights on therapeutic opportunities. Seminars in Cancer Biology, 78, 78–89.
Funding
This work was supported by grants R21-CA235092 (NCI) and W81XWH-20-BCRP-BTA12-2 (DOD) to I.H.G. and by the P30-CA016056 (NCI) Comprehensive Cancer Center grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gelman, I.H. The genomic regulation of metastatic dormancy. Cancer Metastasis Rev 42, 255–276 (2023). https://doi.org/10.1007/s10555-022-10076-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-022-10076-w