Abstract
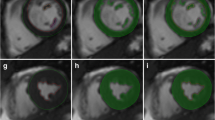
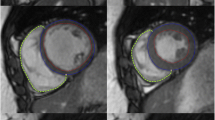
To validate a novel semi-automatic segmentation algorithm for MR-derived volume and function measurements by comparing it with the standard method of manual contour tracing. The new algorithms excludes papillary muscles and trabeculae from the blood pool, while the manual approach includes these objects in the blood pool. An epicardial contour served as input for both methods. Multiphase 2D steady-state free precession short axis images were acquired in 12 subjects with normal heart function and in a dynamic anthropomorphic heart phantom on a 1.5T MR system. In the heart phantom, manually and semi-automatically measured cardiac parameters were compared to the true end-diastolic volume (EDV), end-systolic volume (ESV) and ejection fraction (EF). In the subjects, the semi-automatic method was compared to manual contouring in terms of difference in measured EDV, ESV, EF and myocardial volume (MV). For all measures, intra- and inter-observer agreement was determined. In the heart phantom, EDV and ESV were underestimated for both the semi-automatic. As the papillary muscles were excluded from the blood pool with the semi-automatic method, EDV and ESV were approximately 20 ml lower in the patients, whereas EF was approximately 16 % higher. Intra- and inter-observer agreement was overall improved with the semi-automatic method compared to the manual method. Correlation between manual and semi-automatic measurements was high (EDV: R = 0.99, ESV: R = 0.96; EF: R = 0.80, MV: R = 0.99). The semi-automatic method could exclude endoluminal muscular structures from the blood volume with significantly improved intra- and inter-observer variabilities in cardiac function measurements compared to the conventional, manual method, which includes endoluminal structures in the blood volume.



Similar content being viewed by others
References
Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH (1991) Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med 114(5):345–352
Solomon SD, Anavekar N, Skali H, McMurray JJ, Swedberg K, Yusuf S et al (2005) Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 112(24):3738–3744
Dewey M, Muller M, Eddicks S, Schnapauff D, Teige F, Rutsch W et al (2006) Evaluation of global and regional left ventricular function with 16-slice computed tomography, biplane cineventriculography, and two-dimensional transthoracic echocardiography: comparison with magnetic resonance imaging. J Am Coll Cardiol 48(10):2034–2044
Pattynama PM, Lamb HJ, van der Velde EA, van der Wall EE, de Roos A (1993) Left ventricular measurements with cine and spin-echo MR imaging: a study of reproducibility with variance component analysis. Radiology 187(1):261–268
Sievers B, Kirchberg S, Bakan A, Franken U, Trappe HJ (2004) Impact of papillary muscles in ventricular volume and ejection fraction assessment by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 6(1):9–16
Papavassiliu T, Kuhl HP, Schroder M, Suselbeck T, Bondarenko O, Bohm CK et al (2005) Effect of endocardial trabeculae on left ventricular measurements and measurement reproducibility at cardiovascular MR imaging. Radiology 236(1):57–64
Janik M, Cham MD, Ross MI, Wang Y, Codella N, Min JK et al (2008) Effects of papillary muscles and trabeculae on left ventricular quantification: increased impact of methodological variability in patients with left ventricular hypertrophy. J Hypertens 26(8):1677–1685
Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R (1990) Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 82(2):507–513
Oechslin EN, Attenhofer Jost CH, Rojas JR, Kaufmann PA, Jenni R (2000) Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol 36(2):493–500
Fernandez-Golfin C, Pachon M, Corros C, Bustos A, Cabeza B, Ferreiros J et al (2009) Left ventricular trabeculae: quantification in different cardiac diseases and impact on left ventricular morphological and functional parameters assessed with cardiac magnetic resonance. J Cardiovasc Med (Hagerstown) 10(11):827–833
Codella NCF, Weinsaft JW, Cham MD, Janik M, Prince MR, Wang Y (2008) Left ventricle: automated segmentation by using myocardial effusion threshold reduction and intravoxel computation at MR imaging. Radiology 248(3):1004–1012
Furber A, Balzer P, Cavaro-Menard C, Croue A, Da Costa E, Lethimonnier F et al. (1998) Experimental validation of an automated edge-detection method for a simultaneous determination of the endocardial and epicardial borders in short-axis cardiac MR images: application in normal volunteers. J Magn Reson Imaging 8(5):1006–1014
Graves MJ, Berry E, Eng AA, Westhead M, Black RT, Beacock DJ et al (2000) A multicenter validation of an active contour-based left ventricular analysis technique. J Magn Reson Imaging 12(2):232–239
Lynch M, Ghita O, Whelan PF (2006) Automatic segmentation of the left ventricle cavity and myocardium in MRI data. Comput Biol Med 36(4):389–407
van Geuns RJ, Baks T, Gronenschild EH, Aben JP, Wielopolski PA, Cademartiri F et al (2006) Automatic quantitative left ventricular analysis of cine MR images by using three-dimensional information for contour detection. Radiology 240(1):215–221
van der Geest RJ, Lelieveldt BPF, Angelié E, Danilouchkine M, Swingen C, Sonka M et al (2004) Evaluation of a new method for automated detection of left ventricular boundaries in time series of magnetic resonance images using an active appearance motion model. J Cardiovasc Magn Reson 6(3):609–617
Weinsaft JW, Cham MD, Janik M, Min JK, Henschke CI, Yankelevitz DF et al (2008) Left ventricular papillary muscles and trabeculae are significant determinants of cardiac MRI volumetric measurements: effects on clinical standards in patients with advanced systolic dysfunction. Int J Cardiol 126(3):359–365
Farnebäck G (ed) (2002) Polynomial expansion for orientation and motion estimation. Univ
Otsu N (1975) A threshold selection method from gray-level histograms. Automatica 11:285–296
Spreeuwers L, Bangma S, Meerwaldt R, Vonken E, Breeuwer M (2005) Detection of trabeculae and papillary muscles in cardiac MR images. In: Computers in cardiology. IEEE, pp 415–418
Conflict of interest
K. Jaspers, H.G. Freling, M.J.W. Greuter and T.P. Willems are employees of the department of Radiology of the University Medical Centre Groningen and have no conflict interest to disclose. E.I. Romijn is a student at the department of Physics, Norwegian University of Science and Technology and has no conflict of interest to disclose. C. van Wijk is an employee of Medis and therefore has a potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The semi-automatic segmentation method (QMass MR research edition, Medis, Leiden, The Netherlands) was based on the signal intensity distribution of MR images. The technique of normalized convolution [18] is used to estimate the spatially varying blood and muscle intensities within a user-provided epicardial contour. The voxel intensity is defined by a first order model with six variables:
with:
where a0, a1, a2, b0, b1 and b2 are constants that vary among scans according to the grey value distribution of the image within the epicardial contour. I m(x, y) and I b(x, y) represent the approximation of the intensity of muscle and blood, respectively, at the position (x, y) (see Fig. 4). The constants are obtained with an iterative optimization procedure, during which the weight w(x, y) is initialized to either 1 or 0 using the Otsu threshold method [19].The procedure is stopped when the classification w > 0.5 is unaltered between iterations or when the number of iterations exceeds ten.
Assuming a linear relationship between the fraction of blood and the intensity of the voxel (I(x, y)), the weight w(x, y) represents the fraction of blood in the voxel.
Segmentation procedure. a Shows a short axis view of the heart. The signal intensities along the red line in a are represented in b. The algorithm fits two planes (represented by the solid black lines) through the highest (blood) and lowest (muscle) signal intensities within the epicardium. A threshold plane was defined at 70 % between the two intensity planes. Voxels with signal intensities above this threshold are considered pure blood, and voxels with signal intensities below this threshold are considered to be pure muscle
A binary classification is obtained by thresholding w(x, y). If w(x, y) is higher than the threshold value, the voxel is considered pure blood, otherwise it is defined as pure muscle. In this experiment the threshold value was set to 70 %. Blood volume measures are obtained by multiplying the number of voxels classified as blood with the voxel volume.
Note that this method does not distinct trabeculations from papillary muscle and as such stands out from methods that do specifically target the papillary muscles (See e.g., [20]).
Rights and permissions
About this article
Cite this article
Jaspers, K., Freling, H.G., van Wijk, K. et al. Improving the reproducibility of MR-derived left ventricular volume and function measurements with a semi-automatic threshold-based segmentation algorithm. Int J Cardiovasc Imaging 29, 617–623 (2013). https://doi.org/10.1007/s10554-012-0130-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-012-0130-5