Abstract
Epicardial adipose tissue (EAT) may play an important role for developing an unfavorable cardiovascular risk profile. However, it has not been investigated if EAT is associated with coronary atherosclerosis in non-obese patients. We aimed to evaluate whether EAT is related to coronary atherosclerosis in non-obese patients. Among the consecutive patients who underwent coronary CT angiography (CCTA) with an intermediate pretest likelihood for having coronary artery disease, we excluded the patients whose body mass index (BMI) was over 30 kg/m2 or whose CCTA image quality was not sufficient for the detection of coronary plaque. The remaining patients were divided into the normal and abnormal groups based on the presence of atherosclerotic plaques as seen on the CCTA images. The cardiovascular risk factors were matched between the two groups. Ultimately, 100 patients were included in the normal group and 100 patients were included in the abnormal group. The pericardial fat area was measured at the subaortic level of the heart on the axial CT images. The pericardial fat area was significantly larger in the abnormal group as compared to the normal group (18.1 ± 10.2 vs. 14.6 ± 8.7 cm2, P = 0.019). Even though the overweight patients (25 ≤ BMI < 30) were excluded, the pericardial fat area was still significantly larger in the patients with coronary atherosclerotic plaque as compared to that of the patients without coronary atherosclerotic plaque (18.7 ± 11.5 vs. 13.2 ± 7.9 cm2, P = 0.006). Pericardial fat was more abundant in the non-obese patients with coronary atherosclerosis as compared to that of the patients without coronary atherosclerosis, after controlling for the other cardiovascular risk factors.
Similar content being viewed by others
Introduction
Obesity is an important risk factor for cardiovascular disease (CVD). The regional body adipose tissue distribution, rather than the total body fat, has gained attention as a maker for cardiovascular risk [1–3]. Among the regional deposits of body fat, the abdominal visceral adipose tissue has been the most widely evaluated and this type of adipose tissue has been recognized as a marker for the risk of CVD and metabolic syndrome. Visceral adipose tissue may act as an endocrine and paracrine organ to produce various atherogenic inflammatory cytokines. Visceral adipose tissue accumulation itself may play a role as a major risk factor that contributes to an increased risk of CVD even in non-obese patients [4–7]. Epicardial adipose tissue (EAT) is a particular form of visceral adipose tissue that is deposited around the heart. EAT, as well as abdominal visceral adipose tissue, is a rich source of bioactive adipocytokines that play important roles in the development of atherosclerosis and the occurrence of cardiovascular events [8–15]. EAT is the adipose tissue that directly surrounds the coronary arteries, and this might affect the development of atherosclerosis more than visceral adipose tissue due to EAT’s local effects.
Several studies have shown that the echocardiographically determined EAT thickness on the right ventricle is related to the waist circumference, the abdominal visceral fat, the diastolic blood pressure, the fasting insulin level, the left ventricular mass, insulin resistance and the severity of coronary artery disease (CAD) [9, 12, 16–19]. The use of computed tomography (CT) provides accurate quantification of adipose tissue due to its high spatial resolution as compared to echocardiography. Several methods using CT have demonstrated that the amount of EAT is related to the abdominal visceral adipose tissue, obesity, metabolic syndrome, coronary calcification and CAD [13, 20–22].
Cardiac CT angiography (CCTA) has the ability to evaluate luminal stenosis and to detect atherosclerotic plaques, even in the subclinical stage of CAD. The high spatial resolution of cardiac CT allows accurate measurement of EAT. With the use of CCTA, it is likely that more EAT accumulation may be seen in the subjects with coronary plaque as compared to that of the subjects without coronary plaque, even if the subjects are non-obese. However, the relationship between the EAT that directly surrounds the heart and coronary atherosclerosis in non-obese patients has not yet been investigated. In this study, we have evaluated the correlation between EAT and coronary atherosclerosis in non-obese patients.
Materials and methods
Study population
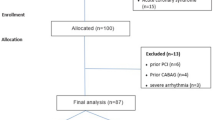
Among the consecutive patients who underwent CCTA and who had an intermediate pretest likelihood of having CAD, we excluded those patients whose body mass index (BMI) was over 30 kg/m2 or whose CCTA image quality was not sufficient for detecting coronary plaque. The remaining patients were divided into the normal and abnormal coronary groups based on whether atherosclerotic plaque was detected on the CCTA images. The cardiovascular risk factors (age, gender, smoking, hypertension, diabetes, dyslipidemia and a history of using medication) were matched in the two groups. Ultimately, 100 patients were included in the normal coronary group and 100 patients were included in the abnormal coronary group.
The smoking behavior, medical history and current cardiovascular drug use (blood pressure-lowering drugs, lipid-lowering drugs and glucose-lowering drugs) were assessed by a questionnaire. Blood sampling was performed to measure the total cholesterol level, triglyceride level, high-density lipoprotein-cholesterol level, low-density lipoprotein-cholesterol level, high-sensitivity C-reactive protein level, fasting blood glucose level and hemoglobin A1C percentage with the patients in an overnight fasting state. Height (m) and body weight (kg) were measured to calculate the body mass index. The systolic and diastolic blood pressures were measured during CT scanning with using an automated and calibrated blood pressure device with the subject in the supine position.
64-slice CT scanning technique
Electrocardiogram (ECG)-gated CCTA studies were performed on a 64 detector-row CT scanner (Brilliance 64, Philips Medical Systems, Cleveland, OH USA). The standard coronary imaging protocols were applied, including the use of an oral beta-blocker (metoprolol) for the patients with heart rates >65 beats/min (unless contraindicated) and nitroglycerin for all the patients. A bolus of 80 ml of contrast agent (Ultravist 370, Schering, Berlin, Germany) was injected intravenously at a rate of 5 ml/s with a subsequently injected 40 ml mixture of saline and contrast agent chase (a ratio of 7:3). As soon as the density in the ascending aorta reached a predefined threshold of 100 Hounsfield units (HU), the scan started automatically with a 6-s scan delay and the entire volume of the heart was scanned during one breath-hold (8–10 s) with the simultaneous recording of the ECG-trace. The imaging parameters were a slice collimation of 64 × 0.625 mm, a gantry rotation time of 420 ms, a tube voltage of 120 kV and a tube current of 800–1,000 mAs/slice. The optimal ECG-phase that provided the best image quality was chosen with using retrospective ECG-gating.
Image analysis
The atherosclerotic plaques were evaluated in all the epicardial segments of the coronary arteries. Structures clearly assignable to the vessel wall (on at least two views) with densities less than the lumen contrast were classified as non-calcified plaque components. Any structure with a density of 130 HU or more that could be visualized separately from the contrast-enhanced coronary lumen was defined as calcified atherosclerotic plaque [23]. A plaque score for each patient was calculated based on the number of diseased segments [23]. The extent of CAD was defined as 0-, 1-, 2-, 3-vessel disease according to the number of coronary vessels with >50% luminal narrowing, as assessed by the gross measurement on the CCTA.
The EAT was measured using the volume analysis software tool of a dedicated workstation (Extended Brilliance Workspace, Philips Medical Systems). Although the previously published methods to measure the volume of the pericardial or epicardial fat can be considered as reference standards [20, 22], these methods were considered to be excessively time and labor intensive. Therefore, we measured the pericardial and epicardial fat volume and area in a subset of 50 patients. The measurements were performed on 1 cm thick axial slices with no interslice-gap. Fat voxels were identified using the threshold attenuation values of −30 to −200 HU. The epicardial fat was separated from the paracardial fat by manual tracing of a single region of interest (ROI) at the pericardium on each slice (Fig. 1). The pericardial and epicardial fat volumes were obtained as the sum of the EAT volume of each 1 cm thick axial slice from the bifurcation level of the main pulmonary artery to the diaphragm. The pericardial and epicardial fat volumes with a 1 cm thickness at the subaortic level of the heart (where the non-coronary cusp of the aortic valve disappeared on the axial image) were regarded as the pericardial and epicardial fat areas, respectively. Measurement of the pericardial fat area at the subaortic level of the heart on a 1 cm thickness axial slice was used for further analysis in the remaining patients. The abdominal visceral adipose tissue area could be measured by the same method as above in 82 patients who underwent abdominal CT or dedicated abdomen fat CT imaging.
Measurement of the pericardial and epicardial fat is described. a The epicardial adipose tissue was measured at the subaortic level of the heart (where the non-coronary cusp of the aortic valve disappeared) on a 1 cm thick axial slice. b The density range between −30 and −200 Hounsfield units was employed to identify the adipose tissue. c Measurement of the pericardial fat area was determined by manual tracing of the mediastinum boundary. d Measurement of the epicardial fat area was determined by manual tracing of the pericardium
Statistical analysis
Continuous variables were computed as means ± standard deviations and the categorical variables were expressed as percentages. The correlation between the EAT measurements and the correlation between the pericardial fat area and the other continuous variables were evaluated by Pearson’s correlation test. The intraobserver and interobserver reproducibility were evaluated by Spearman’s correlation test. The categorical cardiovascular characteristics were compared between the subjects with and without coronary plaque using the chi-square test. The significance of the differences between the mean values of the anthropometric and metabolic variables for the patients with and without coronary atherosclerotic plaque was determined by the use of Student’s unpaired t-test. The correlation between the pericardial fat area and the atherosclerotic plaque score and the severity of CAD was evaluated by Pearson’s correlation test and ANOVA. All the statistical analyses were performed with the statistical software package SPSS for Windows, release 12.0.1 (SPSS, Chicago, IL USA). Associations were considered significant at P values <0.05. All the statistical tests were two-sided.
Results
As previously mentioned, all the measurements of the pericardial and epicardial fat volume and area were performed in a subset of 50 patients. The measurement of the pericardial fat area required approximately 1 min and the measurement of the epicardial fat area required approximately 3 min. For these patients, the analysis showed a good correlation between the pericardial and epicardial fat volume and area measurements (Table 1). The volumetric measurement was time intensive and manual tracing of a ROI at the pericardium was relatively difficult. The intra- and interobserver correlation coefficient of the pericardial fat area was greater than 0.9 (Table 2).
The patients (n = 200) had a mean age of 55.6 ± 12.8 years and the mean body mass index (BMI) was 24.5 ± 2.5 kg/m2 (Table 3). The mean pericardial fat area and the abdominal visceral fat area were 16.4 ± 9.7 and 93.8 ± 59.5 cm2, respectively. The pericardial fat area was correlated with age (r = 0.293, P < 0.001), the high density lipoprotein cholesterol level (r = −0.247, P = 0.006) and the abdominal visceral fat area (r = 0.348, P = 0.001).
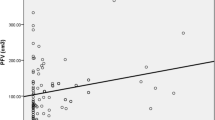
As for the risk factors in the matched groups, those patients with coronary atherosclerotic plaque showed a significantly larger pericardial fat area as compared to that of the patients without coronary plaque (18.1 ± 1.2 vs. 14.6 ± 8.7 cm2, P = 0.019), but the abdominal visceral fat area was similar in these two groups (Table 3). After excluding the overweight patients (25 ≤ BMI < 30), 63 patients were included in the normal coronary group (30 males; mean patient age, 52.5 ± 12.4 years) and 56 patients were included in the abnormal coronary group (33 males; mean patient age, 55.1 ± 13.2 years). The pericardial fat area was still significantly larger in the patients with coronary atherosclerotic plaque as compared to that of the patients without coronary atherosclerotic plaque (18.7 ± 11.5 vs. 13.2 ± 7.9 cm2, P = 0.006). There was also no significant difference of other variables, including the abdominal visceral fat area, according to the presence of coronary atherosclerotic plaque (Table 4). For the overweight patients, the total cholesterol and low-density lipoprotein-cholesterol levels were significantly higher in the abnormal coronary group, but the pericardial fat area was similar for these two groups (Table 5; Fig. 2). The pericardial fat area was correlated with the plaque score and the severity of CAD whether the overweight patients were included or not. However, for the overweight patients, the pericardial fat area displayed no correlation with the plaque score and the severity of the CAD (Table 6).
Discussion
In this case-controlled study, we found that the pericardial fat area at the subaortic level of the heart, as depicted on axial images, shows a relation to the presence of coronary atherosclerotic plaque in non-obese patients after matching the other cardiovascular risk factors. After excluding the overweight patients, the pericardial fat area was also significantly larger in the patients with coronary atherosclerosis as compared to that in the patients without coronary atherosclerosis. The other variables were similar in the two groups. The pericardial fat area showed correlation with the atherosclerotic plaque score and the severity of CAD in all the populations (BMI < 30) and in the normal population (BMI < 25). In the overweight population (25 ≤ BMI < 30), the pericardial fat area was not different between the patients with atherosclerotic plaque and those patients without atherosclerotic plaque and it was not correlated with the plaque score and the severity of CAD. Taguchi et al. [21] have shown that the amount of EAT measured by CT images correlates with the severity of CAD as determined by the use of coronary angiography in non-obese Japanese male patients. Gorter et al. [24] have shown that the amount of EAT is not associated with the severity of coronary atherosclerosis and the coronary artery calcium (CAC) in patients suspected of having CAD. They have also shown that an increased amount of EAT is related to more severe coronary atherosclerosis and CAC in patients with a low BMI (<27 kg/m2), and Greif et al. [25] have shown that the pericardial fat volume is associated with coronary atherosclerosis even after adjusting for BMI in the patients with an intermediate pretest likelihood for CAD. The pericardial fat area was correlated only with age, the high density lipoprotein cholesterol level and the abdominal visceral fat area in the total study population. Previous studies have shown that there is a trend towards a correlation between the amount of EAT and the cardiovascular risk factors. Some investigators have shown that the echocardiographic epicardial fat thickness bears a relation to the waist circumference, the amount of abdominal visceral fat, metabolic syndrome and the left ventricular mass in healthy subjects [16–18], and insulin resistance in obese subjects [19]. Other studies that have used CT imaging have shown that the amount of EAT correlates with the amount of total abdominal visceral adipose tissue in type 2 diabetes patients [20], and with obesity and metabolic syndrome in the patients suspected of having CAD [22]. Our findings relatively agree with those of the previous studies and they suggest that EAT may be more related to the development of coronary atherosclerosis than the traditional cardiovascular risk factors and abdominal visceral adipose tissue even in non-obese subjects. Yet the pericardial fat area was not different between the patients with coronary atherosclerotic plaque and those without coronary atherosclerotic plaque, and the pericardial fat area was not correlated with the plaque score or the severity of CAD in overweight patients, and it was not correlated with many of the traditional cardiovascular risk factors in the total study population. There are some possible explanations for this trend. First, a selection bias may be a reasonable explanation as the study population was based on patients who underwent CCTA. Second, another selection bias may also be a factor as we designed this case controlled-study to include only non-obese patients with excellent CCTA image quality, and the other cardiovascular risk factors were matched. Third, the sample size of the overweight patients in the subgroup analysis might be too small to generate statistical significance.
In this study, we have proposed a new protocol to measure the EAT area at the subaortic level of the heart on the axial cardiac CT images. Some investigators have measured the epicardial fat thickness on the free wall of the right ventricle from both the parasternal long-axis and short-axis views with using echocardiography imaging [9, 11, 12, 16]. However, the use of echocardiography is not the optimal modality for quantification of EAT as differentiating between the epicardial and pericardial fat may be difficult with the use of echocardiography, and the EAT thickness varies at different locations around the myocardium [26]. Furthermore, echocardiographic measurement of the epicardial fat thickness has shown low concordance and poor reproducibility as assessed by the intra-class correlation coefficient [27]. It has recently been emphasized that CT is useful in quantifying EAT accumulation. Taguchi et al. [21] measured the volume of the pericardial fat from the atrial appendage to the apex over the diaphragm with using cross-sectional CT imaging. Wheeler et al. [20] also measured the pericardial fat volume with using a three-dimensional analysis technique. Gorter et al. [22] have stated that the volumetric quantification of EAT with using cardiac CT is highly reproducible as compared to the simpler measurements of the EAT thickness and area. Greif et al. [25] showed that the measurement of the epicardial fat volume was less reproducible as compared to that of the pericardial fat volume. Ding et al. [13] proposed a somewhat simple method by using a protocol to measure the volume of pericardial fat in the region of the heart around the proximal coronary arteries as depicted on cardiac CT scans. de Vos et al. [14] measured the average EAT thickness in the areas of the coronary arteries and they showed that the intra-class correlation coefficient was 0.76. Wang et al. [28] measured the total EAT volume and cross-sectional areas at multiple levels and the EAT thickness at various predefined locations. These investigators showed that the intraobserver and interobserver reproducibility for all the measurements of adipose tissue was greater than 0.95. In this study, there was a good correlation between the pericardial and epicardial fat volumes and areas (Table 1). We measured the EAT area at the subaortic level of the heart where the non-coronary cusp of the aortic valve disappeared on the axial image and the four chambers of the heart were visualized on the axial image. The reason for measuring at the subaortic level was because the partial volume averaging effect seemed to be the least and the subaortic level on the axial images was relatively easy to constantly detect in various subjects without multiplanar reformation. Volumetric quantification of EAT with using CT is a complicated and time consuming procedure and manual tracing of the pericardium is also somewhat difficult. Furthermore, the intra- and interobserver correlation of the pericardial fat area measurement was higher than that of the epicardial fat area. Further, the interobserver correlation of the volumetric measurements was moderate (Table 2). This could be explained by the difficulty of manually tracing the pericardium in some cases, the difficulty of deciding on the bifurcation level of the pulmonary artery and the partial volume averaging effect near the diaphragm. So, we regarded measuring the pericardial fat area as a simple, reliable method for quantification of the EAT. We did not measure the pericardial and epicardial fat thickness, as previous studies have shown the low reproducibility of thickness measurements [14, 27]. Our way of measurement is very simple and more efficient for conducting large epidemiological studies. Investigating the role of EAT in the development of coronary heart disease is possible with the use of CT images, including the non-gated or non-enhanced CT scans such as the low-dose lung cancer screening CT images that are available in several large community-based studies.
This study has several limitations. First, the study population was relatively small and it was exclusively composed of ethnic Koreans who were referred for CCTA. Second, additional measurements, such as the thickness of the EAT, were not performed and not all the EAT measurements were correlated with other risk factors. Some biomarkers that are related with the risk of metabolic syndrome, such as adipokines or insulin, were not investigated. Third, the abdominal visceral fat area was measured in only 82 patients. Fourth, it is also somewhat doubtful that the accuracy of CCTA is sufficient for detecting small non-calcified atherosclerotic plaque. Last, the study design was a retrospective cross-sectional and case-controlled study.
In conclusion, pericardial fat was more abundant in the non-obese patients with coronary atherosclerosis as compared to the non-obese patients without coronary atherosclerosis after controlling for the other cardiovascular risk factors. Our results suggest that local fat deposits around the heart may be more related to the development of coronary atherosclerosis rather than the other cardiovascular risk factors and body fat. Measuring the pericardial fat area at the subaortic level of the heart on axial CT images seems to be an easy and reliable method to evaluate the EAT.
References
Kahn HS, Austin H, Williamson DF et al (1996) Simple anthropometric indices associated with ischemic heart disease. J Clin Epidemiol 49(9):1017–1024
Oppert JM, Charles MA, Thibult N et al (2002) Anthropometric estimates of muscle and fat mass in relation to cardiac and cancer mortality in men: the Paris Prospective Study. Am J Clin Nutr 75(6):1107–1113
Fox CS, Massaro JM, Hoffmann U et al (2007) Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116(1):39–48
Nakamura T, Tokunaga K, Shimomura I et al (1994) Contribution of visceral fat accumulation to the development of coronary artery disease in non-obese men. Atherosclerosis 107(2):239–246
Kobayashi H, Nakamura T, Miyaoka K et al (2001) Visceral fat accumulation contributes to insulin resistance, small-sized low-density lipoprotein, and progression of coronary artery disease in middle-aged non-obese Japanese men. Jpn Circ J 65(3):193–199
Miyawaki T, Abe M, Yahata K et al (2004) Contribution of visceral fat accumulation to the risk factors for atherosclerosis in non-obese Japanese. Intern Med 43(12):1138–1144
Jang Y, Kim OY, Ryu HJ et al (2003) Visceral fat accumulation determines postprandial lipemic response, lipid peroxidation, DNA damage, and endothelial dysfunction in nonobese Korean men. J Lipid Res 44(12):2356–2364
Mazurek T, Zhang L, Zalewski A et al (2003) Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 108(20):2460–2466
Jeong JW, Jeong MH, Yun KH et al (2007) Echocardiographic epicardial fat thickness and coronary artery disease. Circ J 71(4):536–539
Iacobellis G, Pistilli D, Gucciardo M et al (2005) Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine 29(6):251–255
Chaowalit N, Somers VK, Pellikka PA et al (2006) Subepicardial adipose tissue and the presence and severity of coronary artery disease. Atherosclerosis 186(2):354–359
Ahn SG, Lim HS, Joe DY et al (2008) Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart 94(3):e7
Ding J, Kritchevsky SB, Harris TB et al (2008) The association of pericardial fat with calcified coronary plaque. Obesity (Silver Spring) 16(8):1914–1919
de Vos AM, Prokop M, Roos CJ et al (2008) Peri-coronary epicardial adipose tissue is related to cardiovascular risk factors and coronary artery calcification in post-menopausal women. Eur Heart J 29(6):777–783
Baker AR, Silva NF, Quinn DW et al (2006) Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol 5:1
Iacobellis G, Assael F, Ribaudo MC et al (2003) Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes Res 11(2):304–310
Iacobellis G, Ribaudo MC, Assael F et al (2003) Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab 88(11):5163–5168
Iacobellis G, Ribaudo MC, Zappaterreno A et al (2004) Relation between epicardial adipose tissue and left ventricular mass. Am J Cardiol 94(8):1084–1087
Iacobellis G, Leonetti F (2005) Epicardial adipose tissue and insulin resistance in obese subjects. J Clin Endocrinol Metab 90(11):6300–6302
Wheeler GL, Shi R, Beck SR et al (2005) Pericardial and visceral adipose tissues measured volumetrically with computed tomography are highly associated in type 2 diabetic families. Invest Radiol 40(2):97–101
Taguchi R, Takasu J, Itani Y et al (2001) Pericardial fat accumulation in men as a risk factor for coronary artery disease. Atherosclerosis 157(1):203–209
Gorter PM, van Lindert AS, de Vos AM et al (2008) Quantification of epicardial and peri-coronary fat using cardiac computed tomography; reproducibility and relation with obesity and metabolic syndrome in patients suspected of coronary artery disease. Atherosclerosis 197(2):896–903
Leber AW, Becker A, Knez A et al (2006) Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: a comparative study using intravascular ultrasound. J Am Coll Cardiol 47(3):672–677
Gorter PM, de Vos AM, van der Graaf Y et al (2008) Relation of epicardial and pericoronary fat to coronary atherosclerosis and coronary artery calcium in patients undergoing coronary angiography. Am J Cardiol 102(4):380–385
Greif M, Becker A, von Ziegler F et al (2009) Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol 29(5):781–786
Abbara S, Desai JC, Cury RC et al (2006) Mapping epicardial fat with multi-detector computed tomography to facilitate percutaneous transepicardial arrhythmia ablation. Eur J Radiol 57(3):417–422
Saura D, Oliva MJ, Rodriguez D et al (2008) Reproducibility of echocardiographic measurements of epicardial fat thickness. Int J Cardiol [Epub ahead of print]
Wang TD, Lee WJ, Shih FY et al (2009) Relations of epicardial adipose tissue measured by multidetector computed tomography to components of the metabolic syndrome are region-specific and independent of anthropometric indexes and intraabdominal visceral fat. J Clin Endocrinol Metab 94(2):662–669
Acknowledgments
We would like to sincerely thank the radiologic technologist, Sung-Hyun Lee, who participated in the image analysis with the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yong, H.S., Kim, E.J., Seo, H.S. et al. Pericardial fat is more abundant in patients with coronary atherosclerosis and even in the non-obese patients: evaluation with cardiac CT angiography. Int J Cardiovasc Imaging 26 (Suppl 1), 53–62 (2010). https://doi.org/10.1007/s10554-009-9542-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-009-9542-2