Abstract
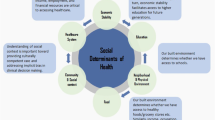
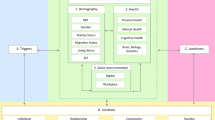
Neighborhoods encompass complex environments comprised of unique economic, physical, and social characteristics that have a profound impact on the residing individual’s health and, collectively, on the community’s wellbeing. Neighborhood disadvantage (ND) is one of several factors that prominently contributes to racial breast cancer (BC) health disparities in American women. African American (AA) women develop more aggressive breast cancer features, such as triple-negative receptor status and more advanced histologic grade and tumor stage, and suffer worse clinical outcomes than European American (EA) women. While the adverse effects of neighborhood disadvantage on health, including increased risk of cancer and decreased longevity, have recently come into focus, the specific molecular mechanisms by which neighborhood disadvantage increases BC risk and worsens BC outcomes (survivorship, recurrence, mortality) are not fully elucidated. This review illuminates the probable biological links between neighborhood disadvantage and predominantly BC risk, with an emphasis on stress reactivity and inflammation, epigenetics and telomere length in response to adverse neighborhood conditions.

Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66(1):7–30
Cronin KA et al (2018) Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer 124(13):2785–2800
Gupta V et al (2018) Racial disparity in breast cancer: can it be mattered for prognosis and therapy. J Cell Commun Signal 12(1):119–132
Howlader N et al (2014) US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. https://doi.org/10.1093/jnci/dju055
Huo D et al (2017) Comparison of breast cancer molecular features and survival by african and european ancestry in the cancer genome atlas. JAMA Oncol 3(12):1654–1662
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics, 2018. CA Cancer J Clin 68(1):7–30
Bauer KR et al (2007) Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer 109(9):1721–1728
Brewster AM, Chavez-MacGregor M, Brown P (2014) Epidemiology, biology, and treatment of triple-negative breast cancer in women of African ancestry. Lancet Oncol 15(13):e625–e634
Cadoo KA, Fornier MN, Morris PG (2013) Biological subtypes of breast cancer: current concepts and implications for recurrence patterns. Q J Nucl Med Mol Imaging 57(4):312–321
Elston CW, Ellis IO (2002) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. C. W. Elston & I. O. Ellis. Histopathology 1991;19:403–410. Histopathology 41(3A):151–152, (discussion 152-153)
Kohler BA, et al (2015) Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst 107(6):djv048
Martin DN et al (2009) Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PLoS ONE 4(2):e4531
Ozdemir BC, Dotto GP (2017) Racial differences in cancer susceptibility and survival: more than the color of the skin? Trends Cancer 3(3):181–197
Chlebowski RT et al (2005) Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst 97(6):439–448
Elledge RM et al (1994) Tumor biologic factors and breast cancer prognosis among white, hispanic, and black women in the United States. J Natl Cancer Inst 86(9):705–712
Akinyemiju TF et al (2015) Residential environment and breast cancer incidence and mortality: a systematic review and meta-analysis. BMC Cancer 15:191
Akinyemiju TF et al (2013) Individual and neighborhood socioeconomic status and healthcare resources in relation to black-white breast cancer survival disparities. J Cancer Epidemiol 2013:490472
Coughlin SS et al (2008) Contextual analysis of breast and cervical cancer screening and factors associated with health care access among United States women, 2002. Soc Sci Med 66(2):260–275
Du XL, Fang S, Meyer TE (2008) Impact of treatment and socioeconomic status on racial disparities in survival among older women with breast cancer. Am J Clin Oncol 31(2):125–132
Elkin EB et al (2010) Geographic access and the use of screening mammography. Med Care 48(4):349–356
DeGuzman PB et al (2017) Impact of urban neighborhood disadvantage on late stage breast cancer diagnosis in virginia. J Urban Health 94(2):199–210
Smith BP, Madak-Erdogan Z (2018) Urban neighborhood and residential factors associated with breast cancer in african american women: a systematic review. Horm Cancer 9(2):71–81
Ellis L et al (2018) Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J Clin Oncol 36(1):25–33
Haji-Jama S et al (2016) Disparities report: disparities among minority women with breast cancer living in impoverished areas of california. Cancer Control 23(2):157–162
Jones A, Paxton RJ (2015) Neighborhood disadvantage, physical activity barriers, and physical activity among african american breast cancer survivors. Prev Med Rep 2:622–627
Kim S, Chukwudozie B, Calhoun E (2013) Sociodemographic characteristics, distance to the clinic, and breast cancer screening results. J Health Dispar Res Pract 6(1):70
Kim S et al (2015) The effects of navigation and types of neighborhoods on timely follow-up of abnormal mammogram among black women. Med Res Arch 1(3):1–10. https://doi.org/10.18103/mra.v0i3.111
Kish JK et al (2014) Racial and ethnic disparities in cancer survival by neighborhood socioeconomic status in Surveillance, Epidemiology, and End Results (SEER) Registries. J Natl Cancer Inst Monogr 2014(49):236–243
Barber S et al (2016) Neighborhood disadvantage, poor social conditions, and cardiovascular disease incidence among african American adults in the jackson heart study. Am J Public Health 106(12):2219–2226
Ross CE, Mirowsky J (2001) Neighborhood disadvantage, disorder, and health. J Health Soc Behav 42(3):258–276
Farmer MM, Ferraro KF (2005) Are racial disparities in health conditional on socioeconomic status? Soc Sci Med 60(1):191–204
Kawachi I, Daniels N, Robinson DE (2005) Health disparities by race and class: why both matter. Health Aff (Millwood) 24(2):343–352
Woods LM, Rachet B, Coleman MP (2006) Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol 17(1):5–19
Ellis L et al (2018) Trends in cancer survival by health insurance status in california from 1997 to 2014. JAMA Oncol 4(3):317–323
Henry KA et al (2013) The joint effects of census tract poverty and geographic access on late-stage breast cancer diagnosis in 10 US States. Health Place 21:110–121
Niu X et al (2013) Cancer survival disparities by health insurance status. Cancer Med 2(3):403–411
Huerta EE et al (2018) Take care of your neighborhood. Breast Cancer Res Treat 167(1):225–234
Henry KA et al (2011) Breast cancer stage at diagnosis: is travel time important? J Commun Health 36(6):933–942
Sampson RJ, Raudenbush SW, Earls F (1997) Neighborhoods and violent crime: a multilevel study of collective efficacy. Science 277(5328):918–924
Fang CY, Tseng M (2018) Ethnic density and cancer: a review of the evidence. Cancer 124(9):1877–1903
Pruitt SL et al (2015) Residential racial segregation and mortality among black, white, and Hispanic urban breast cancer patients in Texas, 1995 to 2009. Cancer 121(11):1845–1855
Bemanian A, Beyer KM (2017) Measures matter: the local exposure/isolation (LEx/Is) metrics and relationships between local-level segregation and Breast cancer survival. Cancer Epidemiol Biomarkers Prev 26(4):516–524
Hill TD, Ross CE, Angel RJ (2005) Neighborhood disorder, psychophysiological distress, and health. J Health Soc Behav 46(2):170–186
Liu YZ, Wang YX, Jiang CL (2017) Inflammation: the common pathway of stress-related diseases. Front Hum Neurosci 11:316
Cohen S, Janicki-Deverts D, Miller GE (2007) Psychological stress and disease. JAMA 298(14):1685–1687
Costanzo ES, Sood AK, Lutgendorf SK (2011) Biobehavioral influences on cancer progression. Immunol Allergy Clin North Am 31(1):109–132
Nagaraja AS et al (2016) SnapShot: stress and disease. Cell Metab 23(2):388–388.e1
Ling J, Kumar R (2012) Crosstalk between NFkB and glucocorticoid signaling: a potential target of breast cancer therapy. Cancer Lett 322(2):119–126
Cash E et al (2015) Circadian disruption and biomarkers of tumor progression in breast cancer patients awaiting surgery. Brain Behav Immun 48:102–114
Fu L, Lee CC (2003) The circadian clock: pacemaker and tumour suppressor. Nat Rev Cancer 3(5):350–361
Agnoli C et al (2017) Biomarkers of inflammation and breast cancer risk: a case–control study nested in the EPIC-Varese cohort. Sci Rep 7(1):12708
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420(6917):860–867
Geng Y et al (2013) Phenotypic switch in blood: effects of pro-inflammatory cytokines on breast cancer cell aggregation and adhesion. PLoS ONE 8(1):e54959
Pierce BL et al (2009) Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol 27(21):3437–3444
Olden K, Olden HA, Lin YS (2015) The role of the epigenome in translating neighborhood disadvantage into health disparities. Curr Environ Health Rep 2(2):163–170
Smith JA et al (2017) Neighborhood characteristics influence DNA methylation of genes involved in stress response and inflammation: the Multi-Ethnic Study of Atherosclerosis. Epigenetics 12(8):662–673
Notterman DA, Mitchell C (2015) Epigenetics and understanding the impact of social determinants of health. Pediatr Clin North Am 62(5):1227–1240
Szyf M (2011) The early life social environment and DNA methylation: DNA methylation mediating the long-term impact of social environments early in life. Epigenetics 6(8):971–978
Chen E et al (2011) Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Mol Psychiatry 16(7):729–737
Kyrtopoulos SA (2013) Making sense of OMICS data in population-based environmental health studies. Environ Mol Mutagen 54(7):468–479
Marshall KW et al (2010) A blood-based biomarker panel for stratifying current risk for colorectal cancer. Int J Cancer 126(5):1177–1186
Esteller M (2008) Epigenetics in cancer. N Engl J Med 358(11):1148–1159
Jaenisch R, Bird A (2003) Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 33(Suppl):245–254
Fleischer T et al (2017) DNA methylation at enhancers identifies distinct breast cancer lineages. Nat Commun 8(1):1379
Kaise M et al (2008) CpG island hypermethylation of tumor-suppressor genes in H. pylori-infected non-neoplastic gastric mucosa is linked with gastric cancer risk. Helicobacter 13(1):35–41
Nakajima T et al (2006) Higher methylation levels in gastric mucosae significantly correlate with higher risk of gastric cancers. Cancer Epidemiol Biomarkers Prev 15(11):2317–2321
Ushijima T (2007) Epigenetic field for cancerization. J Biochem Mol Biol 40(2):142–150
Bediaga NG et al (2010) DNA methylation epigenotypes in breast cancer molecular subtypes. Breast Cancer Res 12(5):R77
Fackler MJ et al (2011) Genome-wide methylation analysis identifies genes specific to breast cancer hormone receptor status and risk of recurrence. Cancer Res 71(19):6195–6207
Fleischer T et al (2014) Genome-wide DNA methylation profiles in progression to in situ and invasive carcinoma of the breast with impact on gene transcription and prognosis. Genome Biol 15(8):435
Holm K et al (2010) Molecular subtypes of breast cancer are associated with characteristic DNA methylation patterns. Breast Cancer Res 12(3):R36
Jovanovic J et al (2010) The epigenetics of breast cancer. Mol Oncol 4(3):242–254
Kamalakaran S et al (2011) DNA methylation patterns in luminal breast cancers differ from non-luminal subtypes and can identify relapse risk independent of other clinical variables. Mol Oncol 5(1):77–92
Ronneberg JA et al (2011) Methylation profiling with a panel of cancer related genes: association with estrogen receptor, TP53 mutation status and expression subtypes in sporadic breast cancer. Mol Oncol 5(1):61–76
van Hoesel AQ et al (2013) Assessment of DNA methylation status in early stages of breast cancer development. Br J Cancer 108(10):2033–2038
Cacioppo JT, Hawkley LC (2003) Social isolation and health, with an emphasis on underlying mechanisms. Perspect Biol Med 46(3 Suppl):S39–S52
Zhang SM et al (2005) Folate intake and risk of breast cancer characterized by hormone receptor status. Cancer Epidemiol Biomarkers Prev 14(8):2004–2008
Boggs DA et al (2010) Fruit and vegetable intake in relation to risk of breast cancer in the Black Women’s Health Study. Am J Epidemiol 172(11):1268–1279
Harris HR et al (2017) An adolescent and early adulthood dietary pattern associated with inflammation and the incidence of breast cancer. Cancer Res 77(5):1179–1187
Gonzalez-Nahm S, et al (2017) Low maternal adherence to a Mediterranean diet is associated with increase in methylation at the MEG3-IG differentially methylated region in female infants. Environ Epigenet 3(2):dvx007
Godfrey KM, Gluckman PD, Hanson MA (2010) Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol Metab 21(4):199–205
Gluckman PD et al (2008) Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359(1):61–73
Boekelheide K et al (2012) Predicting later-life outcomes of early-life exposures. Environ Health Perspect 120(10):1353–1361
McCullough LE et al (2015) Genetic polymorphisms in DNA repair and oxidative stress pathways may modify the association between body size and postmenopausal breast cancer. Ann Epidemiol 25(4):263–269
Rose DP, Haffner SM, Baillargeon J (2007) Adiposity, the metabolic syndrome, and breast cancer in African-American and white American women. Endocr Rev 28(7):763–777
McCullough LE, et al (2015) Gene-specific promoter methylation status in hormone-receptor-positive breast cancer associates with postmenopausal body size and recreational physical activity. Int J Cancer Clin Res 2(1)
Weinsier RL et al (2000) Energy expenditure and free-living physical activity in black and white women: comparison before and after weight loss. Am J Clin Nutr 71(5):1138–1146
Arcidiacono B et al (2012) Insulin resistance and cancer risk: an overview of the pathogenetic mechanisms. Exp Diabetes Res 2012:789174
Henderson KD et al (2006) Ethnic disparity in the relationship between obesity and plasma insulin-like growth factors: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev 15(11):2298–2302
Key TJ et al (2001) Energy balance and cancer: the role of sex hormones. Proc Nutr Soc 60(1):81–89
Muti P (2004) The role of endogenous hormones in the etiology and prevention of breast cancer: the epidemiological evidence. Ann N Y Acad Sci 1028:273–282
Speakman JR, Goran MI (2010) Tissue-specificity and ethnic diversity in obesity-related risk of cancer may be explained by variability in insulin response and insulin signaling pathways. Obesity (Silver Spring) 18(6):1071–1078
Moore SC et al (2016) Association of Leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med 176(6):816–825
McCullough LE et al (2015) Associations between prenatal physical activity, birth weight, and DNA methylation at genomically imprinted domains in a multiethnic newborn cohort. Epigenetics 10(7):597–606
Rodgers KM et al (2018) Environmental chemicals and breast cancer: an updated review of epidemiological literature informed by biological mechanisms. Environ Res 160:152–182
White AJ, Bradshaw PT, Hamra GB (2018) Air pollution and breast cancer: a review. Curr Epidemiol Rep 5(2):92–100
Stults WP, Wei Y (2018) Ambient air emissions of polycyclic aromatic hydrocarbons and female breast cancer incidence in US. Med Oncol 35(6):88
White AJ et al (2016) Sources of polycyclic aromatic hydrocarbons are associated with gene-specific promoter methylation in women with breast cancer. Environ Res 145:93–100
Callahan CL et al (2018) Lifetime exposure to ambient air pollution and methylation of tumor suppressor genes in breast tumors. Environ Res 161:418–424
White AJ et al (2019) Airborne metals and polycyclic aromatic hydrocarbons in relation to mammographic breast density. Breast Cancer Res 21(1):24
Johansson A, Flanagan JM (2017) Epigenome-wide association studies for breast cancer risk and risk factors. Trends Cancer Res 12:19–28
Zhao QY et al (2016) Global histone modification profiling reveals the epigenomic dynamics during malignant transformation in a four-stage breast cancer model. Clin Epigenetics 8:34
Blackburn EH (1991) Structure and function of telomeres. Nature 350(6319):569–573
Monaghan P (2010) Telomeres and life histories: the long and the short of it. Ann N Y Acad Sci 1206:130–142
Olsson M, Wapstra E, Friesen CR (2017) Evolutionary ecology of telomeres: a review. Ann N Y Acad Sci 1422:5–28
von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27(7):339–344
Jennings BJ, Ozanne SE, Hales CN (2000) Nutrition, oxidative damage, telomere shortening, and cellular senescence: individual or connected agents of aging? Mol Genet Metab 71(1–2):32–42
Monaghan P (2014) Organismal stress, telomeres and life histories. J Exp Biol 217(Pt 1):57–66
Barrett EL, Richardson DS (2011) Sex differences in telomeres and lifespan. Aging Cell 10(6):913–921
Campisi J (2005) Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120(4):513–522
Dantzer B, Fletcher QE (2015) Telomeres shorten more slowly in slow-aging wild animals than in fast-aging ones. Exp Gerontol 71:38–47
Harley CB et al (1992) The telomere hypothesis of cellular aging. Exp Gerontol 27(4):375–382
Blackburn EH, Epel ES, Lin J (2015) Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science 350(6265):1193–1198
Koorstra JB et al (2008) Pancreatic carcinogenesis. Pancreatology 8(2):110–125
Londono-Vallejo JA (2008) Telomere instability and cancer. Biochimie 90(1):73–82
Salpea KD et al (2010) Association of telomere length with type 2 diabetes, oxidative stress and UCP2 gene variation. Atherosclerosis 209(1):42–50
Sanders JL, Newman AB (2013) Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiol Rev 35:112–131
Wentzensen IM et al (2011) The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev 20(6):1238–1250
Drury SS et al (2014) The association of telomere length with family violence and disruption. Pediatrics 134(1):e128–e137
Geronimus AT et al (2015) Race-ethnicity, poverty, urban stressors, and telomere length in a detroit community-based sample. J Health Soc Behav 56(2):199–224
Mitchell C et al (2014) Social disadvantage, genetic sensitivity, and children’s telomere length. Proc Natl Acad Sci USA 111(16):5944–5949
Needham BL et al (2015) Leukocyte telomere length and mortality in the National Health and Nutrition Examination Survey, 1999–2002. Epidemiology 26(4):528–535
Park M et al (2015) Where you live may make you old: the association between perceived poor neighborhood quality and leukocyte telomere length. PLoS ONE 10(6):e0128460
Shalev I et al (2013) Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol Psychiatry 18(5):576–581
Theall KP et al (2013) Neighborhood disorder and telomeres: connecting children’s exposure to community level stress and cellular response. Soc Sci Med 85:50–58
Frias C et al (2012) Telomere dysfunction and genome instability. Front Biosci (Landmark Ed) 17:2181–2196
DePinho RA (2000) The age of cancer. Nature 408(6809):248–254
Ennour-Idrissi K, Maunsell E, Diorio C (2017) Telomere length and breast cancer prognosis: a systematic review. Cancer Epidemiol Biomarkers Prev 26(1):3–10
Kammori M et al (2015) Telomere shortening in breast cancer correlates with the pathological features of tumor progression. Oncol Rep 34(2):627–632
Ennour-Idrissi K et al (2016) Association of telomere length with breast cancer prognostic factors. PLoS ONE 11(8):e0161903
Samulin Erdem J et al (2017) Mechanisms of breast cancer risk in shift workers: association of telomere shortening with the duration and intensity of night work. Cancer Med 6(8):1988–1997
Gomez SL et al (2015) The impact of neighborhood social and built environment factors across the cancer continuum: current research, methodological considerations, and future directions. Cancer 121(14):2314–2330
Lynch SM, Rebbeck TR (2013) Bridging the gap between biologic, individual, and macroenvironmental factors in cancer: a multilevel approach. Cancer Epidemiol Biomarkers Prev 22(4):485–495
Warnecke RB et al (2008) Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health 98(9):1608–1615
Zahnd WE, McLafferty SL (2017) Contextual effects and cancer outcomes in the United States: a systematic review of characteristics in multilevel analyses. Ann Epidemiol 27(11):739–748
Polite BN et al (2017) Charting the Future of cancer health disparities research: a position statement from the american association for cancer research, the American Cancer Society, the American Society of Clinical Oncology, and the National Cancer Institute. J Clin Oncol 35(26):3075–3082
Schootman M et al (2017) Geospatial approaches to cancer control and population sciences. Cancer Epidemiol Biomarkers Prev 26(4):472–475
Jackson JW, VanderWeele TJ (2019) Intersectional decomposition analysis with differential exposure, effects, and construct. Soc Sci Med 1:1–10. https://doi.org/10.1016/j.socscimed.2019.01.033
VanderWeele TJ, Jackson JW, Li S (2016) Causal inference and longitudinal data: a case study of religion and mental health. Soc Psychiatry Psychiatr Epidemiol 51(11):1457–1466
Acknowledgments
This work was supported by grants to RA from the National Cancer Institute, including U01 CA179671 and R01 CA169127.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saini, G., Ogden, A., McCullough, L.E. et al. Disadvantaged neighborhoods and racial disparity in breast cancer outcomes: the biological link. Cancer Causes Control 30, 677–686 (2019). https://doi.org/10.1007/s10552-019-01180-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-019-01180-4